Abstract
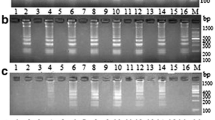
Black-spot shell disease decreases pearl quality and threatens pearl oyster survival. Establishment of a rapid, specific, and sensitive assay to detect Tenacibaculum sp. strain Pbs-1 associated with black-spot shell disease is of commercial importance. We developed a rapid, specific, and highly sensitive loop-mediated isothermal amplification (LAMP) assay to detect Tenacibaculum sp. Pbs-1 in Akoya pearl oysters Pinctada fucata. A set of five specific primers (two inner, two outer, and a loop) were designed based on the 16S–23S internal spacer region of strain Pbs-1. The optimum reaction temperature was 63 °C, and concentrations of the inner and loop primers were 1.4 and 1.0 µM, respectively. The LAMP product can be detected using agarose gel electrophoresis, and the color change in the reaction tube can be detected visually (by the naked eye) following the addition of malachite green. Our assay proved to be specific for strain Pbs-1, with no cross-reactivity with five other species of Tenacibaculum. The detection limit of the LAMP assay at 35 min is 50 pg, and at 60 min it is 5 fg. We evaluated the LAMP assay using diseased and healthy pearl oysters. The results demonstrate the suitability and simplicity of this test for rapid field diagnosis of strain Pbs-1.






Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adamek M, Jung-Schroers V, Hellmann J, Teitge F, Bergmann SM, Runge M, Kleingeld DW, Way K, Stone DM, Steinhagen D (2016) Concentration of carp edema virus (CEV) DNA in koi tissues affected by koi sleepy disease (KSD). Dis Aquat Organ 119:245–251
Atsumi T, Ishikawa T, Inoue N, Ishibashi R, Aoki H, Abe H, Kamiya N, Komaru A (2014) Post-operative care of implanted pearl oysters Pinctada fucata in low salinity seawater improves the quality of pearls. Aquaculture 422:232–238
Barry T, Colleran G, Glennon M, Dunican LK, Cannon F (1991) The 16S/23S ribosomal spacer region as a target for DNA probes to identify eubacteria. PCR Methods Appl 1:51–56
Blomström AL, Hakhverdyan M, Reid SM, Dukes JP, King DP, Belák S, Berg M (2008) A one-step reverse transcriptase loop-mediated isothermal amplification assay for simple and rapid detection of swine vesicular disease virus. J Virol Methods 147:188–193
Bodulev OL, Sakharov IY (2020) Isothermal nucleic acid amplification techniques and their use in bioanalysis. Biochem 85:147–166
Chang DT, Zhang YZ, Ma XY, Zhang W, Wang J (2011) Study on detection of Vibrio parahaemolyticus in shellfish by use of loop-mediated isothermal amplification method. J Food Saf 31:371–378
Compton J (1991) Nucleic acid sequence-based amplification. Nature 350:91–92
Ekman E, Norrgren L (2003) Pathology and immunohistochemistry in three species of salmonids after experimental infection with Flavobacterium psychrophilum. J Fish Dis 26:529–538
En FX, Xiao W, Li J, Chen Q (2008) Loop-mediated isothermal amplification establishment for detection of pseudorabies virus. J Virol Methods 151:35–39
Fahy E, Kwoh DY, Gingeras TR (1991) Self-sustained sequence replication (3SR): an isothermal transcription-based amplification system alternative to PCR. Genome Res 1:25–33
Fall J, Chakraborty G, Kono T, Maeda M, Itami T, Sakai M (2008) Establishment of loop-mediated isothermal amplification method (LAMP) for the detection of Vibrio nigripulchritudo in shrimp. FEMS Microbiol Lett 288:171–177
Fire A, Xu SQ (1995) Rolling replication of short DNA circles. Proc Natl Acad Sci USA 92:4641–4645
Fuertes-Perez S, Hilgarth M, Vogel RF (2020) Development of a rapid detection method for Photobacterium spp. using Loop-mediated isothermal amplification (LAMP). Int J Food Microbiol 334:108805
Gahlawat SK, Ellis AE, Collet B (2009) A sensitive loop-mediated isothermal amplification (LAMP) method for detection of Renibacterium salmoninarum, causative agent of bacterial kidney disease in salmonids. J Fish Dis 32:491–497
Halaihel N, Vendrell D, Ruiz-Zarzuela I, de Blas I, Alonso JL, Gironés O, Pérez T, Muzquiz JL (2009) A new real time PCR-based assay for diagnosing Renibacterium salmoninarum in rainbow trout (Oncorhynchus mykiss) and comparison with other techniques. J Microbiol Methods 76:75–80
Hassan AA, Khan IU, Abdulmawjood A, Lämmler C (2003a) Inter- and intraspecies variations of the 16S–23S rDNA intergenic spacer region of various Streptococcal species. Syst Appl Microbiol 26:97–103
Hassan AA, Vossen A, Lämmler C, Siebert U, Fernández-Garayzábal JF (2003b) PCR amplification of species specific sequences of 16S rDNA and 16S–23S rDNA intergenic spacer region for identification of Streptococcus phocae. Microbiol Res 163:132–135
Hassan AA, Vossen A, Lämmler C, Siebert U, Fernández-Garayzábal JF (2008) PCR amplification of species specific sequences of 16S rDNA and 16S-23S rDNA intergenic spacer region for identification of Streptococcus phocae. Microbiol Res. 163:132–135
Hu H, Zeng W, Wang Y, Wang Q, Bergmann SM, Yin J, Li Y, Chen X, Gao C, Zhang D, Liu C, Ren Y, Shi C (2020) Development and application of a recombinant protein-based indirect ELISA for detection of anti-tilapia lake virus IgM in sera from tilapia. Aquaculture 520:734756
Khimmakthong U, Deshmukh S, Chettri JK, Bojesen AM, Kania PW, Dalsgaard I, Buchmann K (2013) Tissue specific uptake of inactivated and live Yersinia ruckeri in rainbow trout (Oncorhynchus mykiss): visualization by immunohistochemistry and in situ hybridization. Microb Pathog 59–60:33–41
Li Q, Yue Z, Liu H, Liang C, Zheng X, Zhao Y, Chen X, Xiao X, Chen C (2010) Development and evaluation of a loop-mediated isothermal amplification assay for rapid detection of lymphocystis disease virus. J Virol Methods 163:378–384
Liu W, Zhang Y, Ma J, Jiang N, Fan Y, Zhou Y, Cain K, Yi M, Jia K, Wen H, Liu W, Guan W, Zeng L (2020) Determination of a novel parvovirus pathogen associated with massive mortality in adult tilapia. PLoS Pathog 16:e1008765
López JR, Hamman-Khalifa AM, Navas JI, de la Herran R (2011) Characterization of ISR region and development of a PCR assay for rapid detection of the fish pathogen Tenacibaculum soleae. FEMS Microbiol Lett 324:181–188
Lucchi NW, Ljolje D, Silva-Flannery L, Udhayakumar V (2016) Use of malachite green-loop mediated isothermal amplification for detection of Plasmodium spp. Parasites Plos One 11:e0151437
Lutz S, Weber P, Focke M, Faltin B, Hoffmann J, Müller C, Mark D, Roth G, Munday P, Armes N, Piepenburg O, Zengerle R, von Stetten F (2010) Microfluidic lab-on-a-foil for nucleic acid analysis based on isothermal recombinase polymerase amplification (RPA). Lab Chip 10:887–893
Matsuura Y, Terashima S, Takano T, Matsuyama T (2019) Current status of fish vaccines in Japan. Fish Shellfish Immunol 95:236–247
Matsuyama T, Yasuike M, Fujiwara A, Nakamura Y, Takano T, Takeuchi T, Satoh N, Adachi Y, Tsuchihashi Y, Aoki H, Odawara K, Iwanaga S, Kurita J, Kamaishi T, Nakayasu C (2017) A Spirochaete is suggested as the causative agent of Akoya oyster disease by metagenomic analysis. PLoS One. 12: e0182280
Matsuyama T, Miwa S, Mekata T, Matsuura Y, Takano T, Nakayasu C (2021) Mass mortality of pearl oyster (Pinctada fucata (Gould)) in Japan in 2019 and 2020 is caused by an unidentified infectious agent. PeerJ 9:e12180
McCarthy DH (1975) Detection of Aeromonas salmonicida antigen in diseased fish tissue. J Gen Microbiol 88:384–386
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T (2000) Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 28:E63
Osorio CR, Collins MD, Romalde JL, Toranzo AE (2005) Variation in 16S–23S rRNA intergenic spacer regions in Photobacterium damselae: a mosaic-like structure. Appl Environ Microbiol 71:636–645
Piamsomboon P, Jaresitthikunchai J, Hung TQ, Roytrakul S, Wongtavatchai J (2020) Identification of bacterial pathogens in cultured fish with a custom peptide database constructed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). BMC Vet Res 16:52
Reid MS, Le XC, Zhang H (2018) Exponential isothermal amplification of nucleic acids and assays for proteins, cells, small molecules, and enzyme activities. Angew Chem Int Ed Engl 57:11856–11866
Ren W, Renault T, Cai Y, Wang C (2010) Development of a loop-mediated isothermal amplification assay for rapid and sensitive detection of ostreid herpesvirus 1 DNA. J Virol Methods 170:30–36
Ryumae U, Hibi K, Yoshiura Y, Ren H, Endo H (2010) Rapid and highly sensitive detection of Flavobacterium psychrophilum using high gradient immunomagnetic separation with flow cytometry. Aquaculture 309:125–130
Sakatoku A, Fujimura T, Ito M, Takashima S, Isshiki T (2018) Newly isolated bacterium Tenacibaculum sp. strain Pbs-1 from diseased pearl oysters is associated with black-spot shell disease. Aquaculture 493:61–67
Seo H, Kil EJ, Fadhila C, Vo TTB, Auh CK, Lee TK, Lee S (2020) Rapid diagnosis of two marine viruses, red sea bream iridovirus and viral hemorrhagic septicemia virus by PCR combined with lateral flow assay. Virusdisease 31:251–256
Su ZD, Shi CY, Huang J, Shen GM, Li J, Wang SQ, Fan C (2015) Establishment and application of cross-priming isothermal amplification coupled with lateral flow dipstick (CPA-LFD) for rapid and specific detection of red-spotted grouper nervous necrosis virus. Virol J 12:149
Sun ZF, Hu CQ, Ren CH, Shen Q (2006) Sensitive and rapid detection of infectious hypodermal and hematopoietic necrosis virus (IHHNV) in shrimps by loop-mediated isothermal amplification. J Virol Methods 131:41–46
Swain P, Nayak SK (2003) Comparative sensitivity of different serological tests for seromonitoring and surveillance of Edwardsiella tarda infection of Indian major carps. Fish Shellfish Immunol 15:333–340
Vincent M, Xu Y, Kong H (2004) Helicase-dependent isothermal DNA amplification. EMBO Rep 5:795–800
Walker GT, Fraiser MS, Schram JL, Little MC, Nadeau JG, Malinowski DP (1992) Strand displacement amplification-an isothermal, in vitro DNA amplification technique. Nucleic Acids Res 20:1691–1696
Wang D, Wang Y, Zhang M, Zhang Y, Sun J, Song C, Xiao F, Ping Y, Pan C, Hu Y, Wang C, Liu Y (2021) Ladder-shape melting temperature isothermal amplification of nucleic acids. Biotechniques 71:358–369
Wiklund T, Madsen L, Bruun MS, Dalsgaard I (2000) Detection of Flavobacterium psychrophilum from fish tissue and water samples by PCR amplification. J Appl Microbiol 88:299–307
Xu HD, Feng J, Guo ZX, Ou YJ, Wang JY (2010) Detection of red-spotted grouper nervous necrosis virus by loop-mediated isothermal amplification. J Virol Methods 163:123–128
Zhao Y, Chen F, Li Q, Wang L, Fan C (2015) Isothermal amplification of nucleic acids. Chem Rev 115:12491–12545
Acknowledgements
This study was supported in part by grants to A.S. (Grant-in-Aid for Scientific Research [C] No. 20K06203 by JSPS, and Nos. 20E003, 21E018 and 22K008 by Kurita Water and Environment Foundation). We also thank the Edanz Group (https://jp.edanz.com/ac) for editing the language of a draft of this manuscript.
Funding
This study was supported in part by grants to A.S. (Grant-in-Aid for Scientific Research [C] No. 20K06203 by JSPS, and Nos. 20E003, 21E018 and 22K008 by Kurita Water and Environment Foundation).
Author information
Authors and Affiliations
Contributions
Conceptualization—AS and TI; methodology—AS; formal analysis and investigation—TS, YT and MS; writing of original draft—AS; writing and revision—DT, SN and TI; funding acquisition—AS; supervision—AS and TI.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no competing financial interests.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sakatoku, A., Suzuki, T., Tatamiya, Y. et al. Development and evaluation of a rapid, specific, and sensitive loop-mediated isothermal amplification assay to detect Tenacibaculum sp. strain pbs-1 associated with black-spot shell disease in Akoya pearl oysters. Arch Microbiol 205, 43 (2023). https://doi.org/10.1007/s00203-022-03384-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-03384-w