Abstract
Bacillus cereus is a common environmental foodborne microorganism that is mainly found to harbor toxigenic genes with multiple antibiotic resistances and is linked to threatening the safety of dried milk in concern to powdered infant milk formula. In the current investigation, the mean value of B. cereus in 140 samples of powdered milk was 0.57 × 102 ± 0.182 × 102, 0.15 × 102 ± 0.027 × 102, 0.21 × 102 ± 0.035 × 102, and 0.32 × 102 ± 0.072 × 102 CFU/g in a percentage of 64.0 samples of whole milk powder, 43.3 of skim milk powder, 26.7 of powdered infant milk formula and 36.7 milk–cereal-based infant formula, respectively. The results revealed that B. cereus isolates were found to harbor toxigenic genes in the following percentages: 77.8, 2.0, 72.7, 16.2, and 67.7 for nhe, hbl, cytK, ces, and bceT, respectively. Despite all evaluated B. cereus strains were originated from dairy powders, they showed a significant difference (P < 0.05) in their harbored toxigenic cytK gene between whole and skim milk powders with powdered infant formula and milk–cereal-based infant formula, as well as between powdered infant formula and milk–cereal-based infant formula. All isolated B. cereus strains were resistant to cefoxitin, colistin sulfate, neomycin, trimethoprim–sulfamethoxazole, oxacillin, and penicillin. Based on the antimicrobial resistance of B. cereus strains to cephalothin, chloramphenicol, nalidixic acid, and tetracycline, there was a significant difference (P < 0.05) between powdered infant milk formula and whole milk powder strains. This survey is one of few studies proceeded in Egypt to determine the prevalence of toxigenic B. cereus strains in milk–cereal-based infant formula and powdered infant formula as well as skim milk powder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dairy powders are a prevalent product due to their extended usable time with multilateral kind. Infants are massive consumers of powdered milk-based products, as they may potentially in any way be with a weak immune system, especially newborns that did not receive breastfeeding; the passive immunity was not being transferred to them. Baby foods are well known to be the significant nutrition source of choice for kids, especially in the first part of life when they cannot digest other complex food. Their high values for proteins, minerals, fats, and vitamins are undeniable. Infants and babies have the weakest immune system, so the safety and hygienic quality of these baby foods are highly significant in avoiding and controlling their microbial contamination (Rahimi et al. 2013; Sadek et al. 2018).
As declared by the World Health Organization (WHO 2007), B. cereus has now become one of the widespread bacteria that subsist even in pasteurized food products and is categorized as group C that distinguished less danger with the possibility to cause outbreaks in infants by eating infant formula. B. cereus is the most recurrent bacterial contaminant in powdered milk products especially dried infant formula (Di Pinto et al. 2013; Sadek et al. 2018).
The spores of B. cereus are capable of surviving high temperatures and transmitting through heat-treated dairy products. The most common sources of this bacterium are milk powder, and the infant formula industry (Rahimi et al. 2013; Stoeckel et al. 2013; Cetin-Karaca and Morgan 2018). B. cereus is a predominant microorganism, it belongs to B. cereus sensu lato (s.l.) or known as B. cereus group with other genetically linked species as B. anthracis, B. mycoides, B. pseudomycoides, B. thuringiensis, B. cytotoxicus, B. toyonensis, and B. weihenstephanensis (Sánchez-Chica et al. 2020).
The Gram-positive B. cereus organism is responsible for causing diarrhea, emesis, fatal meningitis, tissue destruction, and possibly results in a fatal consequence after contaminated food consumption (Messelhäusser et al. 2007; Frenzel et al. 2015). The reasons for these complications are protein complexes of hemolysin BL (hbl) and non-hemolytic enterotoxin (nhe) genes. Other enterotoxins consist of a single protein encoded for bceT (B. cereus enterotoxin), cytK, as well as one emetic toxin (ces) (Lund et al. 2000; Ehling-Schulz et al. 2006; Hwang and Park 2015).
Multiple antibiotic resistance of B. cereus strains is considered one of its riskiness factors in the treatment of infections as they admitted to being widely resistant to various antimicrobials such as β-lactamase, tetracycline, and quinolones (Godic Torkar and Seme 2009; Ranjbar & Shahreza 2017).
The study purposed to investigate the prevalence of B. cereus, linked species, virulence factors, evaluation of their antibiotic susceptibility, and product acceptability referenced to the Egyptian standards.
Materials and methods
Collection of samples
The samples of 50 whole milk powder, 30 skim milk powder, 30 powdered infant milk formula (for infants from birth till 6 months), and 30 dried milk–cereal-based infant formula (complementary food from 6 month age) were collected from various shops, supermarkets, and pharmacies in Cairo and Giza Governorate, Egypt. These products were imported and repacked in Egyptian factories.
Enumeration and identification B. cereus s.l. based on Guinebretiere et al. (2013) and Bennett et al. (2015)
Twenty-five grams of samples were diluted with 225 ml of 0.1% peptone water (Oxoid, CM0009B) to prepare serial dilutions and mixed in a stomacher (Seward®400) until complete homogenization. Then, 1 ml of first dilution was distributed and spread on four plates of Mannitol Egg Yolk-Polymyxin agar (MYP) (Oxoid, CM0929) as follows: 0.3 ml, 0.3 ml, 0.3 ml, and 0.1 ml. All plates were incubated at 32 °C for 24 h. Five presumptive positive colonies had been selected for confirmation. For all isolates, different biochemical tests have been applied as Gram stain, catalase, nitrate reduction, Voges–Proskauer, anaerobic fermentation of glucose, tyrosine decomposition, and lysozyme resistance to confirm which of them belonged to the B. cereus group and other tests to differentiate species of B. cereus s.l., besides evaluation of virulence factors of B. cereus strains as follows: motility test, rhizoid growth, hemolytic activity, growth at 6 ℃ and 50 ℃, crystal protein staining, and starch hydrolysis test. B. cereus ATCC 14,579 and B. cereus ATCC 11,778 were used as reference strains for the biochemical and molecular tests. After biochemical identification, the count of B. cereus was calculated.
Molecular identification of B. cereus isolates with detection of its toxigenic genes
Extraction of genomic DNA
Genomic DNA was extracted from the culture positively identified as B. cereus using Gene JET genomic DNA purification kit (Thermo Fisher, K0721). The supernatant contains DNA stored at − 20 °C.
PCR for detection of virulence genes
Isolates were tested for gyrB gene by primer pair BC1/BC2r and identified as B. cereus using positive control (ATCC® 14,579™) (Yamada et al. 1999). Besides, testing was performed for the presence of enterotoxigenic genes (nhe, hbl, cytK, and ces) using multiplex PCR according to protocol mentioned by Ehling-Schluz et al. (2006). PCR technique for detection of bceT gene was referenced to Agata et al. (1995). Primer sets’ PCR amplification, details of its sequences, their specific targets, and amplicon sizes were exhibited in Table S1. The amplification cycles were carried out in aPT-100 Thermocycler (MJ Research, USA). PCR amplification products were analyzed and visualized in 1.5% TBE (Tris Borate EDTA) agarose gels under UV light and all PCR experiments were performed twice for each isolate.
Measuring and evaluating antibiotic resistance of B. cereus strains
These were carried out using the protocol of the Kirby–Bauer disk diffusion susceptibility method according to Hudzicki (2009). Fresh Five isolate colonies were picked up and suspended in 2 ml of sterile saline, mixed, and incubated at 37 °C. Then, the turbidity of suspension was adjusted by comparing it with the 0.5 McFarland standard solution. A dipped swab (HiMedia, PW009) from an inoculum tube had used for streaking three times on Muller Hinton agar (MH) (Oxoid, CM0337). The antimicrobial disks were placed and dispensed on the surface of the MH agar using sterile forceps. Finally, the inhibition zone had measured after incubation at 35 °C for 18 h; since interpretive guidelines for B. cereus susceptibility testing are presently not obtainable, the degree of susceptibility of isolates was determined by following the interpretive guidelines for Staphylococcus and other Gram-positive species according to (CLSI 2010; Frenzel et al. 2015). The antimicrobials’ susceptibility disks had used: colistin sulfate, gentamycin, neomycin, tobramycin, and streptomycin (10 μg for each), cefoxitin, cephalothin, chloramphenicol, nalidixic acid, tetracycline and vancomycin (30 μg for each), erythromycin (15 μg), trimethoprim–sulfamethoxazole (25 μg), oxacillin (5 μg) and penicillin (10 U).
Statistical analysis
Results were analyzed statistically by one-way ANOVA and Chi-square independence test using Microsoft Excel 365 enterprise.
Results and discussion
The count of B. cereus was calculated after enumeration and identification of all isolates. Minimum to maximum values of B. cereus in dried samples were 10–8.30 × 102, 10–0.40 × 102, 10–0.30 × 102, and 10–0.80 × 102 CFU/g in 64.0% of whole milk powder, 43.3% skim milk powder, 26.7% powdered infant milk formula and 36.7% milk–cereal-based infant formula, respectively. Based on the mean count of B. cereus, there was a significant difference of (P < 0.05) between whole milk powder and skim milk powder samples, as well as samples of whole milk powder and powdered infant milk formula (Table 1).
These data were closely similar to results obtained by Rahimi et al. (2013), who proved contamination of examined infant cereal-based formula with B. cereus and contributes to the great use of infant food additives or due to the addition of wheat and rice that are rich in starch (Rahimi et al. 2013). Our results were closely related to Aman et al. (2016), who reported B. cereus minimum to a maximum count of 10–9 × 102 CFU/g in 19% of examined infant milk powder. Dried milk products have been notified to be contaminated by high concentrations of B. cereus vegetative cells, and spores include powdered infant milk formula (Stoeckel et al. 2013; Zhang et al. 2017; Cetin-Karaca and Morgan 2018). Spores of B. cereus may enter various dairy products through raw milk (as the soil is the significant source on the farm), and biofilms formed with spores germinate and attach to plant equipment (such as stainless steel) with even resistance to sanitation. Using raw materials of low spore count and improving routine examination to understand the master step during processing at which contamination with spores happened are confirmed to be effective as control and preventive approaches (Stoeckel et al. 2013; Miller et al. 2015; Harada and Nascimento 2021).
As presented in Fig. 1, all species have shown lecithinase zone, while B. cereus and B. thuringiensis were characteristic by hemolytic activity and motility. B. thuringiensis was distinguished by crystal toxin protein formation, while B. mycoides was characterized by rhizoid growth on nutrient agar. However, B. cytotoxicus was identified by the ability to grow at 50 °C, as other members were not able to grow at this temperature. A total of 167 isolates of the B. cereus group were identified as follows: 101 for whole milk powder (53.4% B. cereus, 41.6% B. thuringiensis, 3.0% B. mycoides, and 2.0% B. cytotoxicus), 20 for skim milk powder (80.0% B. cereus and 20.0% B. thuringiensis), 16 for powdered infant milk formula (56.3% B. cereus, 31.2% B. thuringiensis, and 12.5% B. cytotoxicus) and 30 for milk–cereal-based infant formula (66.7% B. cereus, 13.3% B. thuringiensis, 16.7% B. mycoides and 3.3% B. cytotoxicus). Furthermore, Hwang and Park 2015 recognized 41.8% B. cereus and 58.2% B. thuringiensis from 99 powdered infant formula samples.
B. thuringiensis is a common pathogen in milk; it has been stated to produce enterotoxins in food and exhibit cytotoxicity (Johler et al. 2018). However, outbreaks associated with this organism had been discussed in a recent report by EFSA (2016), which declared the insistent demand to further studies to develop a risk assessment of B. thuringiensis in food poisoning outbreaks. While thermo-tolerant B. cytotoxicus has carried the cytotoxin K gene. Besides, it was isolated from milk-based foods for 179 infants that described the possibility of causing outbreaks of food poisoning, which results in 180 considered B. cytotoxicus as a risk factor, especially for neonates (Guinebretiere et al. 2013; Zhang et al. 2017). This species is linked to three deaths of infants in France due to causing necrotic enteritis (Lequin et al. 2005). In addition, infant’s infection with B. cereus had highly increased recently as announced by Frenzel et al. (2015), so EFSA 2016 recommended that this pathogen and its spores in dried milk infant formula must be at least as possible (< 100 CFU/g).
It is highly known that spores of these bacteria after powder milk reconstitution can vegetate, especially with using un-cleaned water or poor equipment sanitization. These unhygienic conditions may result in toxin production in the time of household powder preparation, handling, and during retaining of baby bottles. In addition, polluted ingredients added after drying may cause recontamination or surroundings from drying till packaging (Stoeckel et al. 2013; Cetin-Karaca and Morgan 2018).
B. cereus can produce sphingomyelinase and lecithinase that destroy the membrane of the cell body. They have a hemolytic activity which plays a synergistic role in dissolving red blood cells (RBCs) which also have been associated with multiple outbreaks (Hwang and Park 2015). Consequently, specific tests had performed to determine their capability to express hemolysis and hydrolysis of starch. All isolates had lecithinase activity, and 89.9% of B. cereus isolates were strongly hemolytic. Isolates identified from milk–cereal-based infant formula had the highest percentage of showing hemolysis (95.0%), nearly like data recorded by Sadek et al. (2018). A powerful tool in the pathogenicity of B. cereus is the hydrolysis of starch, which had occurred in 68.7% of isolates. All B. cereus isolates belonging to milk–cereal-based infant formula were productive to starch hydrolysis (Table 2). Hwang and Park 2015 detected the ability of B. cereus to hydrolyze starch in 35.0% of infant formula isolates.
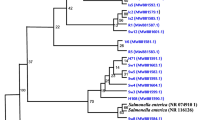
As exhibited in Table 3, a total number of 99 B. cereus isolates were positive for gyrB and confirmed to carry the B. cereus gene as outlined in Fig. S1. Only two isolates recovered from milk–cereal-based infant formula harbored hbl gene, these data approached to finding notified by Sadek et al. (2018). The most excessively distributed gene was nhe in a total percentage of 77.8 as 100.0% for both skim milk powder and powdered infant milk formula. Our results revealed that hbl gene was less prevalent than nhe gene as also demonstrated in previous studies (Hwang and Park 2015; Sadek et al. 2018). Another toxigenic gene is cytK, which is represented by 72.7% of the total examined isolates as 47 of them belonged to whole milk powder samples in a percentage of 87.0. This is the only product that harbored the emetic cereulide toxin gene in a percent (29.6%) by 16 isolates. The cytotoxic gene has hemolysis and necrosis activities on cells and is responsible for causing fatal poisoning outbreaks (Hwang and Park 2015). The cereulide toxin may lead to the severe consequence of damage to the liver, multiorgan dysfunction, and a link to diabetes (Frenzel et al. 2015; EFSA 2016).
As displayed in Figs. S2 and S3, bceT gene that is one of diarrheal enterotoxins was detected in a total percentage of 67.7 of examined isolates and distributed among all products. A total of 41 isolates expressed the 3 toxigenic genes (nhe, cytK and bceT) in the following manner: 22 (40.7%), 10 (62.5%), 3 (33.3%), and 6 (30.0%) isolates from whole milk powder, skim milk powder, powdered infant milk formula, and milk–cereal-based infant formula, respectively, while uniquely 3 (5.6%) from whole milk powder isolates harboring 4 genes (nhe, cytK, ces, and bceT) and 2 (10.0%) from dried milk–cereal-based infant formula were expressed (nhe, cytK, hbl, and bceT). Based on the prevalence of nhe and cytK genes, there was a significant difference (P < 0.05) between B. cereus strains isolated from skim milk powder and milk–cereal-based infant formula. In addition, there was a significance difference between B. cereus strains of whole and skim milk powder for ces and bceT genes, as well as between whole milk powder and milk–cereal-based infant formula for ces and cytK gene.
While based on the prevalence of cytK and bceT genes, there was a significant difference between B. cereus strains isolated from powdered infant milk formula and milk–cereal-based infant formula. Besides a significant difference between B. cereus strains on harboring cytK gene between whole milk powders and powdered infant milk formula, no significant difference was found (P > 0.05) in carrying hbl gene for all isolated strains as shown in Table 3. In this study, B. cereus strains were found to harbor more than toxigenic genes such as nhe, bceT, and/or cytK, so they may have the possibility to result in emetic and diarrheal food poisoning concurrently. Our findings were almost like the research reported by Rahimi et al. (2013), who concluded that 6.7% of B. cereus isolates from dried baby food with milk-based harbored nhe, hbl, and bceT genes.
However, Di pinto et al. (2013) reported comparable to our data, a total of 12 B. cereus strains were isolated from five powdered infant milk formula samples that harbored a minimum one from the following genes: (cytK, hbl, and nhe). While Sadek et al. (2018) had revealed the ability of their isolated B. cereus strains from milk-based baby formula to carry enterotoxigenic genes in the following proportions: 95.5% (43) for cytK gene, 71.1% (32) for nhe, and 11.1% (5) for hbl genes.
The hbl, nhe, and cytK toxigenic genes have caused food poisoning in individuals, as hbl and nhe are responsible for hemolytic and cytotoxic properties, while cytK has been recorded to cause diarrhea with blood (Hwang and Park 2015).
In Fig. 2, strains of B. cereus that carried nhe, hbl, cytK, ces, and bceT were able to show strong hemolysis with starch hydrolysis in a percent of 75.3%, 100.0%, 66.7%, 93.8%, and 71.6%, respectively. These proved the high relation between harboring toxigenic genes and the exhibition of virulence features (lecithinase, strong hemolysis, and starch hydrolysis).
Although some toxigenic strains were weakly hemolytic and could hydrolyze starch, they harbored nhe, cytK, ces, and bceT genes, in percent of 1.2, 1.4, 6.2, and 1.5, respectively. Although some strains were not hydrolyzed starch and exhibited weak hemolysis on blood agar, they could express enterotoxigenic genes in the low percent, 6.6% nhe, 5.5% cytK, and 1.5% bceT gene, while Organji et al. (2015) informed that all B. cereus strains obtained from infant formula milk displayed strong hemolytic character and fewer tendencies to express cytK and hbl.
Pirhonen et al. (2005) announced that B. cereus strains that showed extremely strong hemolysis are the reason for food poisoning. As reported by Andersson et al. (2004), 27.0% of isolated strong hemolytic B. cereus had not produced emetic toxin, while the other 77 isolates demonstrated weak hemolysis with the production of ces toxin.
We assumed a complete association (100.0%) between expressing hbl gene, hemolytic, and starch hydrolytic (Fig. 2). Some researchers announced that the test of starch hydrolysis is an indicator for B. cereus emetic isolates (Ehling-Schulz et al. 2005; Pirhonen et al. 2005), while Hwang and Park (2015) concluded an intense relationship between the ability of B. cereus to hydrolyze starch and the expression of hbl and cytK genes.
In measuring antibiotic sensitivity of the isolated toxigenic B. cereus strains, these belonged to skim milk powder that had shown the most similarity in their pattern and followed by whole milk powder strains. All skim and whole milk powder strains were inhibited by chloramphenicol, gentamycin, nalidixic acid, tetracycline, tobramycin, streptomycin, and vancomycin, and it resisted the cefoxitin, cephalothin, colistin sulfate, neomycin, trimethoprim–sulfamethoxazole, oxacillin, and penicillin (Table 4).
Hundred percent among strains identified from our examined infant foods had resisted cefoxitin, colistin sulfate, neomycin, trimethoprim–sulfamethoxazole, oxacillin, and penicillin antibiotics, while they were susceptible to gentamycin, tobramycin, streptomycin, and vancomycin in a proportion of 100.0%. For remaining antibiotics, these strains had shown different liability to antibiotics in the following manner: 77.8% and 100.0% were resistant to cephalothin for powdered infant milk formula and milk–cereal-based infant formula, respectively, and 88.9% and 15.0% for tetracycline. Exclusively, powdered infant milk formula strains had grown well in the occurrence of chloramphenicol with a percentage of 88.9 and 22.2 for nalidixic acid. Finally, five (25.0%) strains from milk–cereal-based infant formula were resistant to erythromycin and nine (45.0%) to nalidixic acid (Table 4).
Osama et al. (2020) announced that B. cereus isolated from Egyptian dairy products were 100% resistant to colistin, 67.9% resistant to streptomycin, 2.6% resistant to tetracycline, and 5.6% resistant to erythromycin. However, B. cereus strains identified by Kim et al. (2015) showed susceptibility to vancomycin, gentamicin, and tetracycline but impedance to β‐lactam antibiotics such as penicillin and oxacillin.
Ranjbar and Shahreza (2017) presented that resistance of B. cereus from nine milk-based baby food samples that was in a percent of 100, 77.7, 66.6, 44.4, and 11.1 to penicillin, tetracycline, oxacillin, trimethoprim-sulfamethoxazole, and chloramphenicol, respectively.
As presented in Table 4, the resistance of B. cereus strains obtained from powdered infant milk formula to cephalothin and nalidixic acid were significantly different (P < 0.05) with whole milk powder strains. While based on resistance to chloramphenicol and tetracycline, strains from powdered infant milk formula were significantly different (P < 0.05) from whole milk powder, skim milk powder, and milk–cereal-based infant formula strains. As well as based on the results of B. cereus strains’ resistance to erythromycin, there was a significant difference between whole and skim milk powder strains. In addition, there was a significant difference between B. cereus strains isolated from whole milk powder and milk–cereal-based infant formula referred to their resistance to nalidixic acid and tetracycline antibiotics, as well as between skim milk powder and milk–cereal-based infant formula.
With a comparison of B. cereus strains based on antibiotics resistance, strains obtained from powdered infant milk formula and whole milk powder exhibited a higher prevalence statistically significant difference (P < 0.05) than isolated strains from skim milk powder and milk–cereal-based infant formula. There was no significant difference (P > 0.05) in antimicrobial resistance of all obtained B. cereus strains toward cefoxitin, colistin sulfate, gentamycin, neomycin, tobramycin, trimethoprim–sulfamethoxazole, oxacillin, penicillin, streptomycin, and vancomycin.
Several reasons were for expressing drug resistance as the variation of the strain’s origin, transferring of antibiotic resistance, and misusing in treatments. Therefore, it is significant to study the manner of antimicrobial resistance of B. cereus isolated from dairy food with the more restricted policy in the utilization of antimicrobials (Ranjbar and Shahreza 2017; Osama et al. 2020). As deduced from previous results, the B. cereus strains showed resistance to more than one type of antibiotic, so suggested more attention to effective antibiotic therapy to eradicate B. cereus infections. As well as they displayed a significant statistical variation in harboring virulence genes and resistance to antimicrobials, which may be contributing to the fact of having a plastic genome that characterized B. cereus by horizontal gene transmit and results in genetic diversity as reported by Osman et al. (2018).
Referred to Egyptian standards (2006, 2014), the milk powders and infant formula shall be free from pathogenic microorganisms, so samples found to contain B. cereus are considered unacceptable. However, Egyptian standards (2005) announced milk–cereal-based infant formula is acceptable without B. cereus. Consequently, our samples were satisfactory and fit for consumption in the following percentages: 36, 56.7, 73.3, and 63.3 for whole, skim milk powders, powdered infant milk formula, and milk–cereal-based infant formula, respectively, shown in Fig. 3. European Commission (2005) amended a legal limit of B. cereus count of < 50 CFU/g, for powder infant milk formula intended from birth till < 6 month age. Food Standards Australia New Zealand (FSANZ 2004) pronounced B. cereus might reach its infectious dose within 4 h when stored at room temperature with a primary count of 102 CFU/g. One of the products that have a high risk is whole milk powder, especially when reconstituted with cold water as mentioned on its labels, and it mainly depends on the time between preparation and consumption. As documented by EFSA 2016, cells or even spores of B. cereus in a count of > 104 CFU/g will produce diarrheal toxins in the human gut and intestine. Timely manufacturing practice and actualizing food safety management systems are needed for supreme safe production (Ibrahim et al. 2021).
Conclusion
This study shows that B. cereus harbors several toxigenic genes that could contaminate dried milk products, particularly milk formula for pediatrics; this pathogen poses a possible food safety risk. Special attention to the progress of B. cereus antibiotic resistance is required for effective treatment and early recovery. The variation in identified virulence genes and antibiotic resistance between B. cereus strains from different examined samples proved that the type of product was relevant to the count and toxigenicity of B. cereus strains. Consumers should confirm good practices such as proper holding times and storage temperatures. More limitations by Egyptian and international authorities should be applied to control and prevent B. cereus contamination in dried milk for saving low immune system consumers from multiple health problems. Finally, dried milk and powdered infant milk formula should be checked, monitored recurrently, and the application of food safety management systems.
Availability of data and materials
The data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Agata N, Ohta M, Arakawa Y, Mori M (1995) The bceT gene of Bacillus cereus encodes an enterotoxic protein. Microbiology 141(4):983–988. https://doi.org/10.1099/13500872-141-4-983
Aman IM, Abbas EM, Elkassas WM (2016) Safety of infant milk powder sold at Kafrelsheikh governorate markets, Egypt. Int J Innov Res Sci Engin 2:2347–3207. https://doi.org/10.21608/kvmj.2016.108846
Andersson MA, Jääskeläinen EL, Shaheen R, Pirhonen T, Wijnands LM, Salkinoja-Salonen MS (2004) Sperm bioassay for rapid detection of cereulide-producing Bacillus cereus in food and related environments. Int J Food Microbiol 94:175–183. https://doi.org/10.1016/j.ijfoodmicro.2004.01.018
Bennett RW, Tallent SM, Hait JM (2015) Bacillus cereus. In: Salfinger, Y., Tortorello, M.L. (eds) Compendium of Methods for the Microbiological Examination of Foods, 5th edition. American Public Health Association, Washington, DC, USA. Chapter 31, pp. 375–390.
Cetin-Karaca H, Morgan MC (2018) Inactivation of Bacillus cereus spores in infant formula by combination of high pressure and trans-cinnamaldehyde. LWT 97:254–260. https://doi.org/10.1016/j.lwt.2018.07.001
CLSI (2010) Clinical and Laboratory Standards Institute. 2010. Performance standards for antimicrobial susceptibility testing: 20th informational supplement. M100-S20., Wayne, PA.
Di Pinto A, Bonerba E, Bozzo G, Ceci E, Terio V, Tantillo G (2013) Occurence of potentially enterotoxigenic Bacillus cereus in infant milk powder. Eur Food Res Technol 237:275–279. https://doi.org/10.1007/s00217-013-1988-8
EFSA (2016) European food safety authority. Risks for public health related to the presence of Bacillus cereus and other Bacillus spp. including Bacillus thuringiensis in foodstuffs. EFSA J 14(7):4524
Ehling-Schulz M, Vukov N, Schulz A, Shaheen R, Andersson M, Märtlbauer E, Scherer S (2005) Identification and partial characterization of the nonribosomal peptide synthetase gene responsible for cereulide production in emetic Bacillus cereus. Appl Environ Microbiol 71:105–113. https://doi.org/10.1128/aem.71.1.105-113.2005
Ehling-Schulz M, Guinebretiere MH, Monthán A, Berge O, Fricker M, Svensson B (2006) Toxin gene profiling of enterotoxic and emetic Bacillus cereus. FEMS Microbiol Lett 260:232–240. https://doi.org/10.1111/j.1574-6968.2006.00320.x
Egyptian Standards (2005) Processed Cereal-based for foods for Infants and Children. Egyptian Organization for Standardization and Quality Control, Ministry of Industry (ES: 3284/2005)
Egyptian Standards (2006) Infant formula. Egyptian Organization for Standardization and Quality Control, Ministry of Industry (ES: 2072/2006).
Egyptian Standards (2014) Milk powder and cream powder. Egyptian Organization for Standardization and Quality Control, Ministry of Industry (ES: 1780/2014).
European Commission (2005) Commission regulation (EC) No 2073/2005. Official J Eur Union L338:1–26
Frenzel E, Kranzler M, Stark TD, Hofmann T, Ehling-Schulz M (2015) The endospore-forming pathogen Bacillus cereus exploits a small colony variant-based diversification strategy in response to aminoglycoside exposure. Mbio 6(6):e01172-e1215. https://doi.org/10.1128/mbio.01172-15
FSANZ (2004) Food standards Australia New Zealand, Bacillus cereus limits in infant formula. Final Assessment Report, Application, A454.
Godic Torkar K, Seme K (2009) Antimicrobial susceptibility, beta-lactamase and enterotoxin production in Bacillus cereus isolates from clinical and food samples. Folia Microbiol (praha) 54:233–238. https://doi.org/10.1007/s12223-009-0037-2
Guinebretiere MH, Auger S, Galleron N, Contzen M, De Sarrau B, De Buyser ML, Lamberet G, Fagerlund A, Granum PE, Lereclus D, De Vos P, Nguyen-The C, Sorokin A (2013) Bacillus cytotoxicus sp. nov. is a novel thermotolerant species of the Bacillus cereus group occasionally associated with food poisoning. Int J Syst Evol Microbiol 63:31–40. https://doi.org/10.1099/ijs.0.030627-0
Harada AMM, Nascimento MS (2021) Effect of dry sanitizing methods on Bacillus cereus biofilm. Braz J Microbiol 52:919–926. https://doi.org/10.1007/s42770-021-00451-0
Hudzicki J (2009) Kirby-Bauer disk diffusion susceptibility test protocol. Am Soc Microbiol 15:55–63
Hwang J, Park J (2015) Characteristics of enterotoxin distribution, hemolysis, lecithinase, and starch hydrolysis of Bacillus cereus isolated from infant formulas and ready-to-eat foods. J Dairy Sci 98:1–9. https://doi.org/10.3168/jds.2014-9042
Ibrahim AS, Saad MF, Hafiz NM (2021) Safety and quality aspects of whole and skimmed milk powders. Acta Sci Pol Technol Aliment 20(2):165–177. https://doi.org/10.17306/j.afs.0874
Johler S, Kalbhenn EM, Heini N, Brodmann P, Gautsch S, Bağcioğlu M, Contzen M, Stephan R, Ehling-Schulz M (2018) Enterotoxin production of Bacillus thuringiensis isolates from biopesticides, foods, and outbreaks. Front Microbiol 9:1915. https://doi.org/10.3389/fmicb.2018.01915
Kim CW, Cho SH, Kang SH, Park YB, Yoon MH, Lee JB, No WS, Kim JB (2015) Prevalence, genetic diversity, and antibiotic resistance of Bacillus cereus isolated from Korean fermented soybean products. J Food Sci 80:M123–M128. https://doi.org/10.1111/1750-3841.12720
Lequin MH, Vermeulen JR, Van Elburg RM, Barkhof F, Kornelisse RF, Swarte R, Govaert PP (2005) Bacillus cereus meningoencephalitis in preterm infants: neuroimaging characteristics. AJNR Am J Neuroradiol 26:2137–2143
Lund T, De Buyser M, Granum PE (2000) A new cytotoxin from Bacillus cereus that may cause necrotic enteritis. Mol Microbiol 38:254–261. https://doi.org/10.1046/j.1365-2958.2000.02147.x
Messelhäusser U, Fricker M, Ehling-Schulz M, Ziegler H, Elmer-Englhard D, Kleih W, Busch U (2007) Real-time PCR system for the detection of Bacillus cereus (emetic type) in food. J Consum 2:190–193. https://doi.org/10.1007/s00003-007-0172-0
Miller RA, Kent DJ, Watterson MJ, Boor KJ, Martin NH, Wiedmann M (2015) Spore populations among bulk tank raw milk and dairy powders are significantly diff erent. J Dairy Sci 98:8492–8504. https://doi.org/10.3168/jds.2015-9943
Organji SR, Abulreesh HH, Elbanna K, Osman GEH, Khider M (2015) Occurrence and characterization of toxigenic Bacillus cereus in food and infant feces. Asian Pac J Trop Biomed 5:515–520. https://doi.org/10.1016/j.apjtb.2015.04.004
Osama R, Ahmed MFE, Abdulmawjood A, Al-Ashmawy M (2020) Prevalence and antimicrobial resistance of Bacillus cereus in milk and dairy products. Mansoura Vet Med J 21:11–18. https://doi.org/10.35943/mvmj.2020.2.202
Osman KM, Kappell AD, Orabi A et al (2018) Poultry and beef meat as potential seedbeds for antimicrobial resistant enterotoxigenic Bacillus species: a materializing epidemiological and potential severe health hazard. Sci Rep 8(1):11600. https://doi.org/10.1038/s41598-018-29932-3
Pirhonen TI, Andersson MA, Jääskeläinen EL, SalkinojaSalonen MS, Honkanen-Burzalski T, Johansson TML (2005) Biochemical and toxic diversity of Bacillus cereus in a pasta and meat dish associated with a food-poisoning case. Food Microbiol 22:87–91. https://doi.org/10.1016/j.fm.2004.04.002
Rahimi E, Jalali M, Abdos F, Momtaz H, Baghbadorani ZT (2013) Bacillus cereus in infant foods: prevalence study and distribution of enterotoxigenic virulence factors in Isfahan province, Iran. Sci World J 2013:1–5. https://doi.org/10.1155/2013/292571
Ranjbar R, Shahreza MHS (2017) Prevalence, antibiotic-resistance properties and enterotoxin gene profile of Bacillus cereus strains isolated from milk-based baby foods. Trop J Pharm Res 16:1931–1937. https://doi.org/10.4314/tjpr.v16i8.25
Sadek ZI, Abdel-Rahman MA, Azab MS, Darwesh OM, Hassan MS (2018) Microbiological evaluation of infant foods quality and molecular detection of Bacillus cereus toxins relating genes. Toxicol Rep 5:871–877. https://doi.org/10.1016/j.toxrep.2018.08.013
Sánchez-Chica J, Correa MM, Aceves-Diez AE, Castañeda-Sandoval LM (2020) Genetic and toxigenic diversity of Bacillus cereus group isolated from powdered foods. J Food Sci Technol 58(5):1892–1899. https://doi.org/10.1007/s13197-020-04700-2
Stoeckel M, Westermann A, Atamer Z, Hinrichs J (2013) Thermal inactivation of Bacillus cereus spores in infant formula under shear conditions. Dairy Sci Technol 93(2):163–175. https://doi.org/10.1007/s13594-012-0101-6
WHO (2007) World Health Organization. Food safety & food-borne illness. Fact Sheet No. 237, World Health Organization, Geneva, Switzerland.
Yamada S, Ohashi E, Agata N, Venkateswaran K (1999) Cloning and nucleotide sequence analysis of gyrB of Bacillus cereus, B. thuringiensis, B. mycoides, and B. anthracis and their applications to the detection of B. cereus in rice. Appl Environ Microbiol 65(4):1483–1490. https://doi.org/10.1128/aem.65.4.1483-1490.1999
Zhang Y, Chen J, Feng C, Zhan L, Zhang J, Li Y, Yang Y, Chen H, Zhang Z, Zhang Y, Mei L, Li H (2017) Quantitative prevalence, phenotypic and genotypic characteristics of Bacillus cereus isolated from retail infant foods in China. Foodborne Pathog Dis 14(10):564–572. https://doi.org/10.1089/fpd.2017.2287
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No financial support was provided for this study.
Author information
Authors and Affiliations
Contributions
ASI collected samples, carried out the analysis of samples, data analysis, and wrote the manuscript. NMH and MF designed the study, supervised the laboratory work, revised the data analysis, and critically revised all parts of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No competing interests to declare.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ibrahim, A.S., Hafiz, N.M. & Saad, M.F. Prevalence of Bacillus cereus in dairy powders focusing on its toxigenic genes and antimicrobial resistance. Arch Microbiol 204, 339 (2022). https://doi.org/10.1007/s00203-022-02945-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-02945-3