Abstract
The choice of inoculum for successful isolation and establishment of axenic lichen mycobiont culture is a key step towards eliminating endolichenic and lichenicolous fungi and other microbial contamination. The nutritional requirements of each lichen species are unique. This work reports on the isolation, phenotypic plasticity, growth and secondary metabolites from mycobiont culture of the pantropical lichen Platygramme caesiopruinosa. Media composition [Malt yeast extract (MY), Modified Murashige and Skoog (MMS) and Lilly and Barnett (LB) media] was optimized to determine nutritional requirements for optimal growth of this species as assessed by dry biomass and the occurrence of secondary metabolite. Furthermore, the role of different carbon sources in affecting growth, growth stages, phenotypic plasticity, biomass and spectrum of secondary metabolites produced of this mycobiont was examined. The molecular identity of the mycobiont culture was confirmed by amplifying mitochondrial small subunit (mtSSU) sequences. Cultures showed optimum biomass production in MY medium with 10% sucrose. The secondary metabolite profiles for each culture treatment were characterized using High-performance Thin-Layer Chromatography (HPTLC) and Gas Chromatography with Mass Spectrometric (GC–MS) analysis. The HPTLC spectral comparison, phenolic and iodine confirmatory analysis revealed the absence of phenolic metabolites and the presence of non-phenolic metabolites in mycobiont extracts, while GC–MS analysis revealed the biosynthesis of side chain fatty acids, hydrocarbons and sugar alcohol in mycobiont cultures treated with 10% supplemented sucrose as a carbon source.
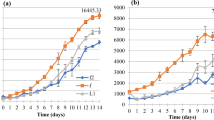
Graphical abstract






Similar content being viewed by others
Abbreviations
- HPTLC:
-
High performance thin layer chromatography
- GC–MS:
-
Gas chromatography with mass spectrometry
- MYC:
-
Mycobiont culture
- MY :
-
Malt yeast extract medium
- MYF:
-
Malt yeast extract medium with fructose
- MYG:
-
Malt yeast extract medium with glucose
- MYM:
-
Malt yeast extract medium with mannitol
- MYR:
-
Malt yeast extract medium with ribitol
- MYS:
-
Malt yeast extract medium with sucrose
- MYSR:
-
Malt yeast extract medium with sorbitol
References
Andrews JH (1992) Fungal life history strategies. In: Carroll GC, Wicklow DT (eds) The fungal community, 2nd edn. Dekker, New York
Arup U, Ekman S, Lindblom L, Mattsson JE (1993) High performance thin layer chromatography (HPTLC), an improved technique for screening lichen substances. Lichenol 25:61–71
Awasthi DD (1991) A key to the microlichens of India, Nepal and Sri Lanka. Biblioth Lichenol 40:1436–1698
Bago B, Cano C, Azcon-Aguilar C, Samson J, Coughlan AP, Piche´ Y, (2004) Differential morphogenesis of the extraradical mycelium of an arbuscular mycorrhizal fungus grown monoexenically on spatially heterogenous culture media. Mycologia 96:452–462
Behera BC, Adawadkar B, Makhija U (2006) Tyrosinase-inhibitory activity in some species of the lichen family Graphidaceae. J Herb Pharmacother 6(1):55–69
Bemmann W (1981) Dimorphism of fungi—review of the literature. Zentralbl Bakteriol Naturwiss 136:369–416
Brunauer G, Hager A, Grube M, Türk R, Stocker Wörgötter E (2007) Alteration in secondary metabolism of aposymbiotically grown mycobionts of Xanthoria elegans and culture resynthesis stages. Plant Physiol Biochem 45:146
Černajová I, Škaloud P (2020) Lessons from culturing lichen soredia. Symbiosis 82:109–122. https://doi.org/10.1007/s13199-020-00718-4
Cordeiro LMC, Iacomini M, Stocker-Wörgötter E (2004) Culture studies and secondary compounds of six Ramalina species. Mycol Res 108:489–497
Crittenden PD, David JC, Hawksworth DL, Campbell FS (1995) Attempted isolation and success in the culturing of a broad spectrum fungi. New Phytol 130:267–297
Culberson CF, Armaleo D (1992) Induction of a complete secondary-product pathway in a cultured lichen fungus. Fungal Genet Biol 16:52
Díaz EM, Zamora JC, Ruibal C et al (2020) Axenic culture and biosynthesis of secondary compounds in lichen symbiotic fungi, the Parmeliaceae. Symbiosis 82:79–93. https://doi.org/10.1007/s13199-020-00719-3
Fazio AT, Bertoni MD, Adler MT et al (2009) Culture studies on the mycobiont isolated from Parmotrema reticulatum (Taylor) Choisy: metabolite production under different conditions. Mycol pro 8:359. https://doi.org/10.1007/s11557-009-0609-1
Fazio AT, Adler M, Maier M (2014) Usnic acid and triacylglycerides production by the cultured lichen mycobiont of Ramalina celastri. Nat Prod Com 9:213–214
Fazio AT, Adler MT, Parnmen S et al (2018) Production of the bioactive pigment elsinochrome A by a cultured mycobiont strain of the lichen Graphis elongata. Mycol pro 17:479–487. https://doi.org/10.1007/s11557-017-1374-1
Gargas A, Taylor JW (1992) Polymerase chain reaction (PCR) primers for amplifying and sequencing nuclear 18S rDNA from lichenized fungi. Mycologia 84:589–592. https://doi.org/10.1080/00275514.1992.12026182
Hamada N (1996) Induction of the production of lichen substances by non-metabolites. Bryologist 99:68–70
Jennings DH (1993) Understanding tolerance to stress: laboratory culture versus environmental actuality. In: Jennings DH (ed) Stress tolerance of fungi. Marcel Dekker, New York
Karthik S (2020) Species diversity, in vitro biosynthesis of secondary metabolites of selected lichen species from habitats in India, and lichen inhabiting-fungal communities of selected lichens from Germany. PhD Thesis, University of Madras, Chennai
Kinoshita Y (1993) The production of lichen substances for pharmaceutical use by lichen tissue culture. Nippon Paint Publications, Osaka
Kukwa M, Schiefelbein U, Flakus A (2013) A contribution to the lichen family Graphidaceae (Ostropales, Ascomycota) of Bolivia. 2. Herzogia 26:231–252. https://doi.org/10.2478/pbj-2014-0017
Le DH, Takenaka Y, Hamada N, Mizushina Y, Tanahashi T (2014) Polyketides from the cultured lichen mycobiont of a Vietnamese Pyrenula sp. J Nat Prod 77:1404–1412
Lilly VG, Barnett HL (1951) Physiology of the fungi. McGraw-Hill, New York
Lücking R, Archer AW, Aptroot A (2009) A world-wide key to the genus Graphis (Ostropales: Graphidaceae). Lichenol 41:363–452. https://doi.org/10.1017/S0024282909008305
Lücking R, Hodkinson BP, Leavitt SD (2017) The 2016 classification of lichenized fungi in the Ascomycota and Basidiomycota—Approaching one thousand genera. Bryologist 119(4):361–416. https://doi.org/10.1639/0007-2745-119.4.361
Lumbsch HT, Leavitt SD (2011) Goodbye morphology? A paradigm shift in the delimitation of species in lichenized fungi. Fungal Divers 50:59. https://doi.org/10.1007/s13225-011-0123-z
McDonald TR, Gaya E, Lutzoni F (2013) Twenty-five cultures of lichenizing fungi available for experimental studies on symbiotic systems. Symbiosis 59:165–171
Miranda-González R, McCune B (2020) The weight of the crust: Biomass of crustose lichens in tropical dry forest represents more than half of foliar biomass. Biotropica 52:1298–1308
Molina M, Crespo A, Vicente C, Elix J (2004) Differences in the composition of phenolics and fatty acids of cultured mycobiont and thallus of Physconia distorta. Plant Physio Biochem. https://doi.org/10.1016/S0981-9428(02)00017-7
Molina MC, Divakar PK, González N (2015) Success in the isolation and axenic culture of Anaptychia ciliaris (Physciaceae, Lecanoromycetes) mycobiont. Mycoscience 56:351–358
Muggia L, Pérez-Ortega S, Fryday A et al (2014) Global assessment of genetic variation and phenotypic plasticity in the lichen-forming species Tephromela atra. Fungal Divers 64:233–251. https://doi.org/10.1007/s13225-013-0271-4
Muthukumar S, Karthik S, Hariharan GN (2016) Mycobiont and whole thallus cultures of Roccella montagnei Bél. for the biosynthesis of secondary compounds. Cryptogam Biodivers Assess. https://doi.org/10.21756/cba.v0i.11014
Orange A, James PW, White FJ (2001) Microchemical methods for the identification of lichens. British Lichen Society, London
Osyczka P, Rola K (2013) Phenotypic plasticity of primary thallus in selected Cladonia species (lichenized Ascomycota: Cladoniaceae). Biologia 68:365–372. https://doi.org/10.2478/s11756-013-0169-3
Pérez-Ortega S, Fernández-Mendoza F, Raggio J, Vivas M, Ascaso C, Sancho L, Los Ríos A (2012) Extreme phenotypic variation in Cetraria aculeata (lichenized Ascomycota), adaptation or incidental modification? Ann Bot 109:1133–1148
Pichler G, Candotto CF, Muggia L, Holzinger A, Tretiach M, Kranner I (2021) Enhanced culturing techniques for the mycobiont isolated from the lichen Xanthoria parietina. Mycol Prog 20:797–808. https://doi.org/10.1007/s11557-021-01707-7
Pittayakhajonwut P, Sri-indrasutdhi V, Dramae A, Lapanun S, Suvannakad R, Tantichareon M (2009) Graphisins A and B from the lichen Graphis tetralocularis. Aust J Chem 62:389–391
Promislow D (2005) A regulatory network analysis of phenotypic plasticity in yeast. Am Nat 165:515–523
Reis RA, Iacomini M, Gorin PAJ, Souza LM, Grube M, Cordeiro LMC, Sassaki GL (2005) Fatty acid composition of the tropical lichen Teloschistes flavicans and its cultivated symbionts. FEMS Microbiol Lett 247:1–6. https://doi.org/10.1016/j.femsle.2005.04.023
Romano AH (1966) Dimorphism. In: Ainsworth GC, Sussman AS (eds) The fungi: An advanced treatise 2. Academic Press, New York, pp 181–209
Sangvichien E, Hawksworth DI, Whalley AJS (2011) Ascospore discharge, germination and culture of fungal partners of tropical lichens, including the use of a novel culture technique. IMA Fungus 2:143–153
Shanmugam K, Srinivasan M, Hariharan GN (2016) Developmental stages and secondary compound biosynthesis of mycobiont and whole thallus cultures of Buellia subsororioides. Mycol Prog 15:41
Singh P, Singh KP (2014) Two new species of Graphis (Ascomycota: Ostropales: Graphidaceae), from Indo-Burma biodiversity hotspot. Mycosphere 5:504–509. https://doi.org/10.5943/mycosphere/5/4/2
Singh KP, Sinha GP (2010) Indian lichens: an annotated checklist. Botanical Survey of India, Kolkata
Slepecky RA, Starmer WT (2009) Phenotypic plasticity in fungi: a review with observations on Aureobasidium pullulans. Mycologia 101(6):823–832. https://doi.org/10.3852/08-197
Srinivasan M, Shanmugam K, Kedike B, Narayanan S, Shanmugam S, Gopalasamudram Neelakantan H (2020) Trypethelone and phenalenone derivatives isolated from the mycobiont culture of Trypethelium eluteriae Spreng and their anti-mycobacterial properties. Nat Prod Res 34(23):3320–3327. https://doi.org/10.1080/14786419.2019.1566823
Stocker-Wörgötter E (2007) Polyketides and PKS genes in lichen-forming fungi. The impact of algal transfer metabolites (polyols and glucose) on the production of “lichen substances”. (SEB Main Meeting, Symbiosis, Glasgow: 31.3.-4.4.2007). Comp Biochem Physiol (Part A) 146(2007):215–223
Stocker-Wörgötter E (2008) Metabolic diversity of lichen-forming ascomycetous fungi: culturing, polyketide and shikimate metabolite production, and PKS genes. Nat Prod Rep 25(1):188–200. https://doi.org/10.1039/b606983p
Stocker-Wörgötter E, Elix JA (2006) Morphogenetic strategies and induction of secondary metabolite biosynthesis in cultured lichen forming ascomycota, as exemplified by Cladia retipora (Labill.) Nyl. and Dactylina arctica (Richards) Nyl Symbiosis 41:9–20
Stomp M, van Dijk MA, van Overzee HMJ, Wortel MT, Sigon CAM, Egas M, Hoogvel H, Grons HJ, Huisman J (2008) The time scale of phenotypic plasticity and its impact on competition in fluctuating environments. Am Nat 172:169–185
Takenaka Y, Hamada N, Tanahashi T (2008) Biosynthetic origin of graphenone in cultured lichen mycobionts of Graphis handelii. Zeitschrift für Naturforschung 63C:565–568
Takenaka Y, Morimoto N, Hamada N, Tanahashi T (2011) Phenolic compounds from the cultured mycobionts of Graphis proserpens. Phytochemistry 72:1431–1435. https://doi.org/10.1016/j.phytochem.2011.04.017
Takenaka Y, Naito Y, Le DH, Hamada N, Tanahashi T (2013) Napthoquinones and phenaleone derivatives from the cultured mycobiont of Trypethelium sp. Heterocycles 87:1897–1902
Tanahashi T, Kuroishi M, Kuwahara A, Nagakura N, Hamada N (1997) Four phenolics from the cultured lichen mycobionts of Graphis scripta var. pulverulenta. Chem Pharm Bull 45:1183–1185
Tanahashi T, Takenaka Y, Nagakura N, Hamada N, Miyawaki H (2000) Two isocoumarins from the cultured lichen mycobiont of Graphis sp. Heterocycles 53:723–728
Tanahashi T, Takenaka Y, Nagakura N, Hamada N (2003) 6H Dibenzo[b,d]pyran-6-one derivatives from the cultured lichen mycobionts of Graphis spp. and their biosynthetic origin. Phytochemistry 62(1):71–75. https://doi.org/10.1016/S0031-9422(02)00402-8
Tretiach M, Brown DH (1995) Morphological and physiological differences between epilithic and epiphytic populations of the lichen Parmelia pastillifera. Ann Bot 75:627–632
Valarmathi R, Hariharan GN (2007) Soredial culture of Dirinaria applanata (Fée) Awasthi: observations on developmental stages and compound production. Symbiosis 43:137–142
Valarmathi R, Hariharan GN, Gayatri V, Ajay P (2009) Characterization of a non-reducing polyketide synthase gene from lichen Dirinaria applanata. Phytochemistry 70:721–729. https://doi.org/10.1016/j.phytochem.2009.04.007
Valarmathi R (2009) Bioprospecting secondary compounds from selected lichen species in vitro production, characterization and molecular basis of compound production. PhD thesis, University of Madras, Chennai
Vallardes F, Gianoli E, Gómez JM (2007) Ecological limits to plant phenotypic plasticity. New Phytol 167:749–776
Vo V, Le DH, Thanh NT, Nguyen NH, Vo TP, Sichaem J, Nguyen VK, Duong TH (2020) A new eremophilane-sesquiterpene from the cultured lichen mycobiont of Graphis sp. Nat Prod Res. https://doi.org/10.1080/14786419.2020.1779717
Williams L, Colesie C, Ullmann A, Westberg M, Wedin M, Büdel B (2017) Lichen acclimation to changing environments: Photobiont switching vs. climate—specific uniqueness in Psora decipiens. Ecol Evol 7:2560–2574
Yamamoto Y, Mizuguchi R, Takayama S, Yamada Y (1987) Effects of culture conditions on the growth of Usneaceae lichen tissue cultures. Plant Cell Physiol 28:1421–1426
Zoller S, Scheidegger C, Sperisen C et al (1999) PCR primers for the amplification of mitochondrial small subunit ribosomal DNA of lichen-forming ascomycetes. Lichenol 31(5):511–515
Acknowledgements
Prof. M.S. Swaminathan, Founder Chairman of M.S. Swaminathan Research Foundation (MSSRF) and the Executive Director (MSSRF) M.S. Swaminathan Research Foundation, are thanked for their unwavering encouragement and support. Dr. Gayatri Venkataraman, MSSRF and Suni Sebastian, for correcting the language; Mr. G.K. Dayanandham and S. Kannapan assisted the authors in revising figures, formatting and uploading the final version of the manuscript. The authors are grateful for the financial support provided by the Department of Biotechnology, Government of India.
Author information
Authors and Affiliations
Contributions
KS and MS collected the lichen samples, conceptualized and conducted the in vitro experiments, secondary metabolites profiling and data analysis and wrote the original draft. GNH supervised the experiments, corrected and approved the manuscript for publishing.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shanmugam, K., Srinivasan, M. & Neelakantan, H.G. Insights into in vitro phenotypic plasticity, growth and secondary metabolites of the mycobiont isolated from the lichen Platygramme caesiopruinosa. Arch Microbiol 204, 90 (2022). https://doi.org/10.1007/s00203-021-02685-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-021-02685-w