Abstract
Summary
The refracture rate after major trauma is approximately half (57%) the refracture rate after a minimal trauma injury.
Extending Fracture Liaison Service activity to include major trauma patients creates significant additional direct cost, but remains essentially cost neutral if notional savings through refracture risk reduction are taken into account.
Purpose
To compare the 3-year refracture rate following minimal trauma (MT) and non-minimal trauma (non-MT) injuries and evaluate the cost of extending fracture liaison service (FLS) operations to non-MT presentations.
Methods
Patients aged 50, or above presenting to the John Hunter Hospital with a fracture in calendar year 2018 were identified through the Integrated Patient Management System (IPMS) of the Hunter New England Health Service’s (HNEHS), and re-presentation to any HNEHS facility over the following 3 years monitored.
The refracture rate of MT and non-MT presentations was compared and analysed using Cox proportional hazards regression models. The cost of including non-MT patients was estimated through the use of a previously conducted micro-costing analysis. The operational fidelity of the FLS to the previous estimate was confirmed by comparing the 3-year refracture rate of MT presentations in the two studies.
Results
The 3-year refracture rate following a MT injury was 8% and after non-MT injury 4.5%. Extension of FLS activities to include non-MT patients in 2022 would have cost an additional $198,326 AUD with a notional loss/saving of $ − 26,625/ + 26,913 AUD through refracture risk reduction. No clinically available characteristic at presentation predictive of increased refracture risk was identified.
Conclusion
The 3-year refracture after a non-MT injury is about half (57%) that of the refracture rate after a MT injury. Extending FLS activity to non-MT patients incurs a significant additional direct cost but remains cost neutral if notional savings gained through reduction in refracture risk are taken into account.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients who suffer a minimal trauma (MT) fracture constitute the highest risk group for further fragility fractures [1,2,3,4,5]. For this reason, organisations such as the International Osteoporosis Foundation (IOF) advocate for the creation of fracture liaison service (FLS) to identify these patients in order to intervene, reduce the refracture risk, and stop the fracture ‘cascade’ [6,7,8,9].
FLS operates on the basis of reviewing hospital records to identify these patients and as a rule exclude patients whose fracture had been the result of a major injury—a non-MT fracture. The question remains whether this non-MT group may also harbour patients with increased risk of refracture and are inappropriately excluded from what may be important evaluation and intervention [10, 11].
A recent study by Leslie et al. suggested that this may indeed be the case [12]. In a retrospective study of 14,758 patients over 40 years of age who had a bone mineral density (BMD) evaluation at baseline, they found no significant difference in the subsequent major refracture rate between patients whose index fracture had been due to high trauma versus those with low trauma, though the cumulative incidence was higher in the low trauma group. Mean Z-scores for those with index high trauma fractures were significantly lower than those who had no previous fractures and were similar to patients who had index low trauma fractures.
We had previously reported on the effectiveness of our FLS and showed a relative reduction of 40% in the risk of refracture of a major bone over 3 years with a NNP (number needed to process) of 25 [13]—an effect size superior to statin therapy for ischaemic heart disease (NNT 55) [14], and greater than the relative risk reduction achieved in controlled studies of commonly used antiresorptive drugs [15].
The service is based on skilled nurses reviewing patients presenting to the John Hunter Hospital and initiating the relevant follow-up steps if the patient is judged to have sustained their fracture as a result of a minimal trauma event. Those outside these parameters are excluded.
The current study was undertaken to explore the effect of this approach by comparing the 3-year refracture rate between MT and non-MT presentations.
As our FLS operates under constrained resources and funding, we also sought to ascertain the additional cost and resource utilisation burden that would be generated by including the non-MT patients.
As in a typical case captured by our FLS, there is no information about their BMD prior to the presenting fracture, we also sought to identify any characteristic that might indicate increased risk of refracture.
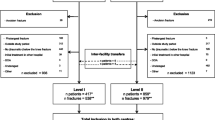
To estimate the likely cost we confirmed the fidelity of the FLS by comparing the refracture rate in MT patients in the current cohort and those we reported previously and utilised this [13] for the related detailed costing analysis [16].
Methods
Study population
The study cohort consisted of patients presenting to John Hunter Hospital Emergency Department (JHH ED) from January to December 2018. John Hunter Hospital (JHH) is a tertiary referral public hospital in Newcastle, New South Wales, Australia. It services the Hunter New England Local Health District, covering a region of 131,785 km2 with 38 peripheral hospitals. In 2021 the estimated population size of this region was 962,390 residents [17].
Data collection
The ICD-10 codes for fractures, described by S02.0 (fracture of skull) to S92.9 (fracture of foot), were used to identify all patients presenting to JHH ED with a fracture between 1st January 2018 and 31st December 2018.
All fractures passing through the JHH ED are reviewed by the JHH fracture liaison service nurses who determine eligibility for inclusion into the FLS programme. The initial mechanism of injury for these individuals is coded by the FLS nurses by reviewing their electronic medical record at presentation. Minimal trauma (MT) fractures were classified as a “fall from standing height or less”, “bending over”, “incidental finding on X-ray”, “inversion/eversion”, “sneezing/coughing”, or “spontaneous”. Pathological fractures and those without a clear mechanism of injury were classified as “Other” and were excluded prior to analysis. The remaining fractures were classified as non-minimal trauma (non-MT) fractures. Data regarding the index fracture (number and location of fracture(s)) was extracted from the electronic medical record, and the types of fracture (major bone vs minor bone) were recorded. Hip, spine, femur, pelvis, and humerus fractures were denoted as major bones. Fractures involving all other bones were classified as minor bones.
All patients presenting to a Hunter New England Health Service (HNEHS) facility with a fracture over the following 3 years (1st January 2018–31st December 2020) were identified and classified using ICD-10 codes. Individual level patient data was used to determine the number of re-presentations of the 2018 cohort within the 3-year period. All multiple presentations were manually reviewed and merged if they occurred within a 21-day time frame for the same individual. The date of death (if applicable) was obtained via the Hunter New England Local Health District Patient Administration System (iPM).
Costing
To calculate the direct cost of incorporating non-MT fractures into the FLS programme and the projected savings to the health system from the reduced refracture rate, we utilised our earlier microcosting analysis of the JHH FLS [16], with values adjusted for a yearly inflation of 2.4% [18]. Processing costs related to “fracture capture” for MT and non-MT patients were assumed to be the same. For possible differences in direct osteoporosis treatment costs a sensitivity analysis was done, looking at 10, 25, 50, 75, or 100% of the non-MT patients receiving direct anti-osteoporosis treatment. Projected savings through 3-year refracture prevention was based on the previously published cost of fracture management [16]. Based on the approximately 50% lower refracture rate in the non-MT group (see results) a commensurate 50% reduction was applied to applied to the projected cost saving. No adjustment was made for possible later changes in fracture management costs.
Fine and Grey proportional hazards models were used to analyse the risk of refracture [19, 20]. The primary outcome for this model was the time to bone refracture, where death from any cause was considered a competing risk. A series of sensitivity analyses were conducted to explore relationships between characteristics of the index fracture and the refracture. The likelihood of major and minor refractures as well as the impact of multiple fractures on refracture rates was also analysed. Logistic regression models were used to assess the associations between the index refracture characteristics in patients who experienced a refracture. All statistical tests were conducted at a 5% significance value. Models included age, sex, and whether the incident fracture was a major or minor fracture. All data processing and analysis was performed using R statistical software v4.3.1 [21].
The study was approved by the Hunter New England Human Research Ethics Committee.
Results
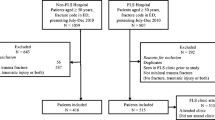
During calendar year 2018, 1541 patients aged 50 years and above presented to JHH ED with a fracture. One thousand twenty (66.2%) were minimal trauma fractures. Four hundred eighty-eight (31.7%) were non-minimal trauma fractures, and 33 (2.1%) were not in either category (pathological fractures, or those with mechanism of injury not adequately recorded) and were excluded from analysis. Within the 3-year follow-up period, there were 20,010 emergency department fracture presentations at all HNEHS facilities. Upon extracting the relevant data points, 104 (6.9%) out of 1510 index patients had refractured at least once. 400 (26.5%) patients died during the time frame of the study.
There were several differences in the baseline characteristics of the MT and non-MT cohorts (Table 1).
The non-MT cohort was significantly younger than the MT cohort with 60.5% under the age of 65 compared to 25.1% in the MT cohort (p < 0.001), and had a lower rate of death during follow-up (8.4% vs 35.2% in the MT cohort). The non-MT cohort was majority male (59.8%), whereas the MT cohort was majority female (73.8%) (p < 0.001).
A total of 80.5% of non-MT patients had a minor fracture as the index fracture, compared to 52.6% of MT patients (Table 1).
In the MT cohort, 82 out of 1020 patients refractured (8%), while 22 of the 488 non-MT patients experienced a refracture (4.5%). Patients with incident non-MT fracture had a 44% lower risk of refracture compared to the MT cohort (HR = 0.55, 95% CI 0.34, 0.88, p = 0.012). After correcting for age and fracture type as potential confounders, this effect remained (HR 0.62, 95% CI 0.38, 0.1, p = 0.05) (Table 2).
Index fracture types
A total of 70.7% of refractures were of a major bone if the index fracture had been of a major bone (Table 3). In pooled analysis, the odds of refracture being of a major bone were significantly higher in patients whose index fracture had been of a major bone (HR 5.60, 95% CI: 2.42, 13.66, p < 0.001) (Table 4).
Similarly, if the index fracture was of a minor bone, in 69.8% the refracture was also of a minor bone.
This relationship persisted in sub-analysis of MT and non-MT groups, though sample sizes were smaller (82 and 22, respectively).
There was no significant difference between incident major and minor fractures and the likelihood of refracture.
Gender and age
There was no significant gender-related effect on the risk of refracture in the non-MT cohort (HR 0.81, 95% CI: 0.35, 1.86, p = 0.61) or the MT cohort (HR 0.9, 95% CI: 0.55, 1.49, p = 0.69) in proportional hazards regression models. Similarly, age (< or ≥ 65) was not associated with the risk of refracture for non-MT (HR 0.71, 95% CI: 0.29,1.73, p = 0.45) or for MT (HR1.65, 95% CI: 0.93, 2.94, p = 0.088).
Number of index fractures
There was no significant difference between the number of index fractures (one bone vs more than one bone) and the likelihood of refracture (HR = 0.63, 95% CI: 0.28, 1.43, p = 0.27).
Costing
Based on the 2015 microcosting determination of processing 1000 MT patients [16], the addition of 488 non-MT patients would have resulted in an equivalent additional direct cost of $144,788–198,326 AUS in 2022 dollars to the $406,407 cost of processing the 1000 MT patients with a projected notional loss of $26,625 to a potential saving of $26,913 to the health system through a reduced number of refractures (Table 5).
Discussion
The IOF and similar organisations have advocated fracture liaison services as the preferred way of responding to the anticipated rise in osteoporotic fractures projected to be of a magnitude capable of overwhelming health systems [6,7,8,9].
The FLS approach is based on identifying patients at the highest risk of fracture and in whom intervention can be expected to have the greatest impact. The model involves case finding whereby patients over the age of 50 with a fracture after a minimal trauma injury, by the fact of their fracture have self-identified as being at very high risk of further fractures. The model generally excludes patients whose fracture had been the result of major trauma. The desirability of this exclusion has been repeatedly questioned since 1993 and most recently in 2021 [12, 22,23,24,25,26,27].
The JHH has operated a Type A FLS [28] since 2007, with a demonstrated impact on refracture risk [13, 29].
To explore the feasibility and cost of expanding the service to include non-MT fractures, we determined the refracture rate of MT and non-MT presentations and estimated the likely cost and potential saving to the health system.
Several studies have shown that the non-MT group is likely to harbour a significant percentage of patients with known osteoporosis [12, 22,23,24,25,26,27].
FLS operates on the basis of identifying patients who present with a fracture. At the time of presentation; however, information about their bone health is seldom available as a potential guide to their future fracture risk and selection for in-depth assessment.
In a comparative analysis of the 3-year refracture in patients over the age of 50, we found the risk of refracture to be 44% lower following a non-MT fracture than a MT fracture, with an absolute risk of refracture of 4.5% in the non-MT group.
Though lower than the MT group, the 4.5% refracture rate in the non-MT group nevertheless carries significant disability and cost implications, especially as one in five (19.5%) of the refractures are of major bones.
We were unable to identify readily accessible characteristics as an indicator of increased refracture risk that would enable focus on a higher risk subgroup among non-MT patients and potentially reduce the extra work of processing the non-MT patients. Counterintuitively the data did not show a relationship between age (HR 0.71, 95% CI: 0.29,1.73, p = 0.45) or gender (HR 0.81, 95% CI: 0.35, 1.86, p = 0.61) and the risk of refracture.
While fracture of a major bone in both the non-MT and the MT group was a strong indicator that the refracture would also involve a major bone, neither the type nor the number of index fractures was predictive of increased refracture risk.
We were able to estimate the cost of extending the activity of our FLS to include non-MT cases by utilising the detailed costing data that we had previously determined [16]. To confirm the validity of this approach, we compared the current FLS activity with the previous evaluation. We confirmed the fidelity between the two analyses by showing that the 3-year refracture in MT patients has remained the same [13].
We estimated the cost of processing the additional 488 patients who had an incident non-MT fracture in the 12-month study period to be between $144,788 and $198,326 AUS (adjusted to 2022 dollars) depending on the percentage receiving direct anti-osteoporosis treatment.
By exclusively targeting the 19.5% of patients whose index fracture involved a major bone and at risk that the refracture would also be of a major bone the additional cost could be reduced from $198,326 to $38,673.
In terms of opportunity cost savings for the health system through fractures prevented, we estimate a net saving of $13,528 (2022 dollars) if direct anti-osteoporosis treatment was confined to the 25% of patients likely to be osteoporotic by bone mineral density assessment as found in an earlier Sydney based study [25]. A net loss of $26,625 however, is likely if all non-MT patients were to receive direct anti-osteoporosis treatment. While the most likely scenario of 25% receiving direct anti-osteoporosis treatment therefore represents a small potential net gain for the health system, the gain is significantly less than the most stringent costing model for MT fractures [18].
A strength of our study is that utilising the information base of an established FLS serving a defined catchment area we were able to analyse a large number of fracture events and follow them up over a 3-year period, through a central electronic medical record system.
We were also able to compare the fracture–refracture characteristics of the FLS during the study period with an earlier detailed analysis of the FLS’s effectiveness and operational characteristics.
The rate of leakage (presentation on refracture to non-HNELHD facilities such as general practitioners, different health networks, and out of catchment area health services) however was not actively determined for the study, and in the absence of any significant change in population characteristics or health service organisation was assumed to be the same as in the previous study.
Important limitations of the study include the lack of contemporaneous information about fracture rate in the general population.
Further, the refracture rate is the observed incidence over 3 years after the index fracture creating a distortion in that MT fracture presentations are subject to the benefits of the FLS whereas non-MT presentations are actively excluded.
We had previously documented that the JHH FLS has led to a 40% relative and 5% absolute reduction in the 3-year refracture rate [13]. While analysis of the refracture of the current MT group confirms maintenance of FLS effectiveness, estimates of likely refracture risk without the FLS remain an extrapolation. Similarly, we have no information about any osteoporosis assessment and treatment in the non-MT group post the index fracture event and the impact of the FLS on the refracture rate in the non-MT group is a pro-rata extrapolation from the demonstrated effect in the MT group, rather than a direct observation, and may in practice be different.
The estimated cost saving through the reduction in refracture incidence is also an extrapolation based on the 2015 costs of fracture management. Although adjusted for inflation it does not take into account cost changes due to possible changes in fracture management.
In conclusion, we found the extension of the FLS to include non-MT patients would be at a significant additional operational cost and a diluted opportunity cost saving to the health system.
How this is seen by funding sources and the effect on funding decisions is likely to be greatly dependent on the payment arrangements within the particular health system.
In the system at the JHH, and the system generally in Australia, the expense of running a FLS is borne by the hospital out of the annual general budget granted to the hospital through the state department of health without specific funds being directed for FLS use.
The benefit in savings from reduced expenses and resource utilisation through prevented fractures however accrues to the health system as a whole, and in particular to the Commonwealth (national) Department of Health, which allocates funds to the states to run public hospitals.
At the hospital level, however, fund and resource allocation is largely an exercise of balancing a range of competing needs where the potential opportunity benefits to the broader health system is just one of the considerations.
References
Center JR, Bliuc D, Nguyen TV, Eisman JA (2007) Risk of subsequent fracture after low-trauma fracture in men and women. JAMA: J Am Med Assoc 297(4):387–394. https://doi.org/10.1001/jama.297.4.387
Cummings SR, Melton LJ (2002) Epidemiology and outcomes of osteoporotic fractures. Lancet 359(9319):1761–1767. https://doi.org/10.1016/S0140-6736(02)08657-9
Kanis JA, Johnell O, De Laet C, Johansson H, Oden A, Delmas P, Eisman J, Fujiwara S, Garnero P, Kroger H, McCloskey EV, Mellstrom D, Melton LJ, Pols H, Reeve J, Silman A, Tenenhouse A (2004) A meta-analysis of previous fracture and subsequent fracture risk. Bone 35(2):375–382. https://doi.org/10.1016/j.bone.2004.03.024
van Staa TP, Leufkens HGM, Cooper C (2002) Does a fracture at one site predict later fractures at other sites? A British cohort study. Osteoporos Int: A J Established Result Cooperation Between Eur Found Osteoporos Natl Osteoporos Found USA 13(8):624–629. https://doi.org/10.1007/s001980200084
Frost SA, Kelly A, Gaudin J, Evoy LM, Wilson C, Marov L, El Haddad C, Center J, Eisman JA, Nguyen TV, Hassett G (2020) Establishing baseline absolute risk of subsequent fracture among adults presenting to hospital with a minimal-trauma-fracture. BMC Musculoskelet Disord 21(1):133. https://doi.org/10.1186/s12891-020-3161-4
Akesson K, Marsh D, Mitchell PJ, McLellan AR, Stenmark J, Pierroz DD, Kyer C, Cooper C, IOF Fracture Working Group (2013) Capture the fracture: a best practice framework and global campaign to break the fragility fracture cycle. Osteoporos Int: A J Established Result Cooperation Between Eur Found Osteoporos Natl Osteoporos Found USA 24(8):2135–2152. https://doi.org/10.1007/s00198-013-2348-z
Eisman JA, Bogoch ER, Dell R, Harrington JT, McKinney RE Jr, McLellan A, Mitchell PJ, Silverman S, Singleton R, Siris E, ASBMR Task Force on Secondary Fracture Prevention (2012) Making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. J Bone Mineral Res: Off J Am Soc Bone Mineral Res 27(10):2039–2046. https://doi.org/10.1002/jbmr.1698
Marsh D, IOF CSA Fracture Working Group, Åkesson K, Beaton DE, Bogoch ER, Boonen S, Brandi M-L, McLellan AR, Mitchell PJ, Sale JEM, Wahl DA (2011) Coordinator-based systems for secondary prevention in fragility fracture patients. Osteoporos Int: A J Established Result Cooperation Between Eur Found Osteoporos Natl Osteoporos Found USA 22(7):2051–2065. https://doi.org/10.1007/s00198-011-1642-x
Binkley N, Blank RD, Leslie WD, Lewiecki EM, Eisman JA, Bilezikian JP (2017) Osteoporosis in crisis: it’s time to focus on fracture: Osteoporosis in crisis. J Bone Min Res 32(7):1391–1394. https://doi.org/10.1002/jbmr.3182
Cummings SR, Eastell R (2020) Stop (mis)classifying fractures as high- or low-trauma or as fragility fractures. Osteoporosis Int 31(6):1023–1024. https://doi.org/10.1007/s00198-020-05325-z
Schafer AL, Shoback DM (2021) A distinction without a difference-does it matter whether fractures are nontraumatic or traumatic? JAMA Intern Med 181(8):1063–1064. https://doi.org/10.1001/jamainternmed.2021.2599
Leslie WD, Schousboe JT, Morin SN, Martineau P, Lix LM, Johansson H, McCloskey EV, Harvey NC, Kanis JA (2020) Fracture risk following high-trauma versus low-trauma fracture: a registry-based cohort study. Osteoporos Int 31(6):1059–1067. https://doi.org/10.1007/s00198-019-05274-2
Nakayama A, Major G, Holliday E, Attia J, Bogduk N (2016) Evidence of effectiveness of a fracture liaison service to reduce the re-fracture rate. Osteoporos Int 27(3):873–879. https://doi.org/10.1007/s00198-015-3443-0
Taylor F, Huffman MD, Macedo AF, Moore THM, Burke M, Davey Smith G, Ward K, Ebrahim S (2013) Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2021(1):CD004816. https://doi.org/10.1002/14651858.CD004816.pub5
Cranney A, Guyatt G, Griffith L, Wells G, Tugwell P, Rosen C, Osteoporosis Methodology Group and The Osteoporosis Research Advisory Group (2002) Meta-analyses of therapies for postmenopausal osteoporosis. IX: summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocr Rev 23(4):570–578. https://doi.org/10.1210/er.2001-9002
Major G, Ling R, Searles A, Niddrie F, Kelly A, Holliday E, Attia J, Bogduk N (2019) The costs of confronting osteoporosis: cost study of an Australian fracture liaison service: Costs of confronting osteoporosis. JBMR Plus 3(1):56–63
Australian Bureau of Statistics (2016) Hunter 2016 All persons Quickstats. The Australian Government
Reserve bank of Australia (n.d.) Inflation Calculator
Fine JP, Gray RJ (1999) A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94(446):496. https://doi.org/10.2307/2670170
Nolan EK, Chen H-Y (2020) A comparison of the Cox model to the Fine-Gray model for survival analyses of re-fracture rates. Arch Osteoporos 15(1):86. https://doi.org/10.1007/s11657-020-00748-x
R core team (n.d.) R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria
Karlsson MK, Hasserius R, Obrant KJ (1993) Individuals who sustain non osteoporotic fractures continue to also sustain fragility fractures. Calcif Tissue Int 53(4):229–231
Sanders KM, Pasco JA, Ugoni AM, Nicholson GC, Seeman E, Martin TJ, Skoric B, Panahi S, Kotowicz MA (1998) The exclusion of high trauma fractures may underestimate the prevalence of bone fragility fractures in the community: the Geelong Osteoporosis Study. J Bone Min Res 13(8):1337–1342. https://doi.org/10.1359/jbmr.1998.13.8.1337
Mackey DC, Lui L-Y, Cawthon PM, Bauer DC, Nevitt MC, Cauley JA, Hillier TA, Lewis CE, Barrett-Connor E, Cummings SR, Study of Osteoporotic Fractures (SOF) and Osteoporotic Fractures in Men Study (MrOS) Research Groups (2007) High-trauma fractures and low bone mineral density in older women and men. JAMA 298(20):2381–2388. https://doi.org/10.1001/jama.298.20.2381
Pereira L, Bliuc D, Stanford P, Eisman JA, Center JR (2017) More-than-minimal-trauma fractures are associated with low bone density: an 8-year prospective study. Osteoporos Int 28(1):103–110. https://doi.org/10.1007/s00198-016-3739-8
Muschitz C, Kocijan R, Baierl A, Dormann R, Feichtinger X, Haschka J, Szivak M, Muschitz GK, Schanda J, Pietschmann P, Resch H, Dimai HP (2017) Preceding and subsequent high- and low-trauma fracture patterns-a 13-year epidemiological study in females and males in Austria. Osteoporos Int 28(5):1609–1618. https://doi.org/10.1007/s00198-017-3925-3
Crandall CJ, Larson JC, LaCroix AZ, Robbins JA, Wactawski-Wende J, Johnson KC, Sattari M, Stone KL, Weitlauf JC, Gure TR, Cauley JA (2021) Risk of subsequent fractures in postmenopausal women after nontraumatic vs traumatic fractures. JAMA Intern Med 181(8):1055–1063. https://doi.org/10.1001/jamainternmed.2021.2617
Ganda K, Puech M, Chen JS, Speerin R, Bleasel J, Center JR, Eisman JA, March L, Seibel MJ (2013) Models of care for the secondary prevention of osteoporotic fractures: a systematic review and meta-analysis. Osteoporos Int 24(2):393–406. https://doi.org/10.1007/s00198-012-2090-y
Van der Kallen J, Giles M, Cooper K et al (2014) A fracture prevention service reduces further fractures two years after incident minimal trauma fracture. Int J Rheum Dis 17(2):195–203
Acknowledgements
The authors would like to thank Dr Ayano Kelly for helpful comments in the preparation of this study.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Chandrasoma, D., Chiu, S., Niddrie, F. et al. Should major trauma fractures be part of a fracture liaison service’s remit: a cost–benefit estimate. Osteoporos Int (2024). https://doi.org/10.1007/s00198-024-07134-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00198-024-07134-0