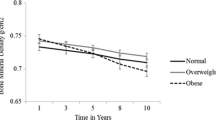
Abstract
Summary
There are limited longitudinal data regarding relationships between changes in body composition and bone mineral density (BMD). In 3671 participants aged 46-70 years at baseline, ∆lean mass was a stronger determinant than ∆fat mass of ∆BMD over 6 years. Maintained or increased lean mass may slow down age-related bone loss.
Purpose
There are limited longitudinal data regarding relationships between changes in body composition and bone mineral density (BMD) with ageing. We examined these in the Busselton Healthy Ageing Study.
Methods
We studied 3671 participants (2019 females) aged 46-70 years at baseline with body composition and BMD assessments by dual-energy x-ray absorptiometry at baseline and after ~6 years. Relationships between changes in total body mass (∆TM), lean mass (∆LM) and fat mass (∆FM) with ∆BMD at total hip, femoral neck and lumbar spine were evaluated using restricted cubic spline modelling (accounting for baseline covariates) and mid-quartile least square means were compared.
Results
∆TM was positively associated with ∆BMD of total hip and femoral neck in both sexes, and spine in females; in females but not males, associations plateaued at ∆TM above ~5kg for all sites. In females, ∆LM was positively associated with ∆BMD of all three sites with plateauing of the relationship at ∆LM above ~1kg. Women in the highest quartile of ∆LM (Q4, mid-quartile value +1.6 kg) had 0.019-0.028 g/cm2 less reduction in BMD than those in the lowest quartile (Q1, -2.1 kg). In males, ∆LM was positively associated with ∆BMD of total hip and femoral neck; men in Q4 (+1.6 kg) had 0.015 and 0.011 g/cm2 less bone loss, respectively, compared with Q1 (-2.7 kg). ∆FM was positively associated with ∆BMD of total hip only in both sexes.
Conclusion
∆LM is a stronger determinant than ∆FM of ∆BMD. Maintained or increased LM is associated with less age-related bone loss.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis and associated fractures present a major public health challenge [1]. Body mass is an important determinant of bone mineral density (BMD) [2], due to the associated mechanical loading to the bone [3], and low body weight is a well-recognised risk factor for fracture [4]. On the other hand, obesity is associated with increased cardiometabolic risk [5] and in middle-aged adults we reported that the positive relationships between body mass index (BMI) and BMD were weaker at high BMI (i.e. when BMI above 30-35 kg/m2) [6]. In addition, a longitudinal cohort study showed that older adults with baseline BMI in the obesity category had greater annual loss of femoral neck BMD compared with participants with normal BMI, indicating that high body mass is not necessarily protective against bone loss [7].
Randomised controlled trials (RCTs) of caloric restriction have shown that intervention induced weight loss is related to a decrease in BMD [8], but weight regain after the trial does not increase BMD [9]. In the Women's Health Initiative study, compared with stable body weight, weight gain, weight loss, and intentional weight loss were associated with increased incidence of fracture at different locations [10]. Regarding the components of body mass, cross-sectional studies showed that in men and pre-menopausal women, lean mass is a stronger predictor than fat mass for bone mass, whereas in postmenopausal women, the influence of lean mass and fat mass on BMD were comparable [11,12,13]. Few studies have evaluated the longitudinal relationships between change in body composition and BMD during middle to older age. In a study with 172 men and 165 women aged over 65 years, rate of loss of lean mass in the lower limbs was positively associated with the rate of total hip BMD loss in women, although the associations were attenuated when rate of loss in leg muscle strength was included in the model (P =0.07) [14]. Associations between change in fat mass and BMD were not evaluated in that study. In 539 participants of the Study of Women’s Health Across the Nation, during menopause transition (defined as the four years surrounding the final menstrual period) greater loss of lean mass was associated lower femoral neck BMD and greater gain in fat mass was associated with higher femoral neck and lumbar spine BMD at the end of the transition period [15].
In a cross-sectional analysis of participants in the Busselton Healthy Ageing Study (BHAS) at baseline, we previously reported positive relationships between lean and fat mass with BMD, which were attenuated or absent in individuals with higher BMI [6]. With the collection of further dual-energy x-ray absorptiometry (DXA) body composition and BMD data after ~6 years follow-up, the aim of the present study was to perform a longitudinal analysis of associations between changes in total body mass, lean mass and fat mass with changes in BMD of total hip, femoral neck and lumbar spine in this representative cohort of middle-to-older aged Australians.
Participants and methods
Participants
The Busselton Healthy Ageing Study [16] is a longitudinal cohort study of “Baby Boomers” (born between 1946 and 1964 inclusive) who live within the City of Busselton local government boundary, in south-western Western Australia, which has a predominantly white population. All non-institutionalised residents listed on the electoral roll were invited to participate (in Australia, electoral roll registration is compulsory). At baseline (Phase 1, May 2010 to December 2015), 5107 participants were recruited, representing ~80% of those eligible. The 6-year survey (Phase 2) was conducted between March 2016 and January 2022, and 3888 participants (76%) attended. Of these, 3692 participants had valid DXA BMD and body composition assessment at both baseline and 6-year. After exclusion of 21 participants taking antiresorptive agents at baseline, 3671 (2019 females) were included in this analysis. The study was approved by the University of Western Australia Human Research Ethics Committee (Number RA/4/1/2203). Written informed consent was obtained from participants.
DXA scans
At baseline and 6 years, BMD (g/cm2) of total hip, femoral neck and anterior-posterior lumbar spine (L1-L4) and body composition (whole body lean and fat mass) were measured by DXA using a GE Lunar Prodigy Pro densitometer (Madison, WI, USA) [17]. Scans were analysed using enCORE Version 16 software (GE Health) with the “copy” feature used to analyse follow-up scans; manual inspection of regions of interest and adjustment where necessary were made by two independent reviewers (KZ and MH). Calibration of the DXA scanner using a phantom was performed prior to each scanning session according to manufacturer’s recommendations. For the duration of the study, there was no obvious shift in the phantom BMD values on the quality assurance plot (coefficient of variation = 0.30%). Precision error was less than 2.0% for all measured sites based on repeated scans in a random sample of 30 subjects.
Other assessments
At baseline and 6 years, body mass and height were measured by standard anthropometric techniques and BMI calculated as body mass (kg) / height (m)2. At baseline, demographic data were collected by questionnaire, including race, education level, smoking habit, alcohol consumption (glasses per day), medical history, and medication and supplement use [16]. The International Physical Activity Questionnaire (IPAQ) was used to assess physical activity and sedentary behaviour, with physical activity level categorised as low, medium and high according to the IPAQ scoring protocol [18]. Venous blood samples were collected after an overnight fast, and serum 25-hydroxyvitamin D (25OHD) measured using the ARCHITECT 25-OH Vitamin D immunoassay (Abbott Laboratories, Abbott Park, Illinois, US) [17].
Data analysis
Variables are presented as means ± standard deviations (SD) for descriptive statistics or means (95% confidence internal) or means ± standard error of mean (SEM) for estimated (adjusted) values unless otherwise stated. Comparisons of characteristics between males and females were made by Student's t-test and chi-squared test.
Restricted cubic spline modelling, which allows the assessment of whether the relationship is non-linear, was used to evaluate the relationships between change in total, lean and fat mass with change in bone measures over 6 years, with change in BMD of each site [∆BMD, 6 years – baseline] as the dependent variable and changes in total mass, lean mass or fat mass [∆TM, ∆LM or ∆FM, 6 years – baseline] as independent variables using R package “rms” with three knots (10th, 50th and 90th percentile) [19]. Covariates adjusted in the models were race, measurement interval between baseline and follow-up DXA assessment, and baseline BMD value, age, total mass (or lean and fat mass for the models for ∆LM or ∆FM), height, smoking history (never/previous/current), serum 25OHD, physical activity level (low/medium/high), sitting hours per day, alcohol consumption (glasses per day), education level, calcium supplementation and use of menopausal hormone treatment (MHT, in women); models for ∆LM or ∆FM adjusted for each other. Due to the sex difference in body composition and its age-related change [20], males and females were analysed separately. The least square means of ∆BMD of each site at mid-quartile levels of each of ∆TM, ∆LM or ∆FM were estimated, and comparisons between mid-quartile means were made.
In a further analysis, participants whose body mass was stable from baseline to 6 years (change in total body mass <3% [21]) were grouped by whether LM and FM increased or decreased over time, as Group 1 (↓LM & ↓FM), Group 2 (↓LM & ↑FM), Group 3 (↑LM & ↓FM) and Group 4 (↑LM & ↑FM), and comparisons for ∆TM and ∆BMD between groups made by general linear model. The models for ∆TM adjusted for baseline TM, the models for ∆BMD adjusted for baseline BMD value, lean mass, fat mass, and calcium supplementation, and all the models adjusted for race, measurement interval, and baseline age, height, smoking history, serum 25OHD, physical activity level, sitting hours per day, alcohol consumption, education level and MHT (in women). Statistical significance level was set at P < 0.05 (two-tailed). All analyses were performed using IBM SPSS (version 27, IBM, Chicago, IL, USA) and R (version 4.0.3, R Foundation for Statistical Computing, Vienna, Austria).
Results
Characteristics of study participants
In total, 3671 participants were included in this analysis, and Fig. 1 depicts how the study was sample derived. Compared with those who attended the baseline survey but were not included in this analysis (n = 1436), those included were not significantly different in proportion of females (55.0% vs 54.5%, P = 0.73) and baseline age (57.9 ± 5.7 vs 58.2 ± 5.9 years, P = 0.09), but had lower BMI (28.0 ± 4.7 vs 28.8 ± 5.5 kg/m2, P < 0.001), higher proportion of high physical activity level (46.2% vs 43.1%, P = 0.009) and lower proportion of current smoker (7.8% vs 16.0%, P < 0.001) at baseline.
Table 1 shows participant characteristics at baseline and changes in body composition and BMD over 6 years. Mean age at baseline was 57.9 ± 5.7 years, and mean measurement interval between baseline and follow-up 6.2 ± 0.9 years. There were no significant differences in mean change in total mass (∆TM, 0.3 ± 5.7 vs 0.1 ± 5.2 kg) and fat mass (∆FM, 0.7 ± 4.7 vs 0.6 ± 4.2 kg) between females and males. However, males had greater loss in lean mass compared with females (∆LM, −0.6 ± 2.1 vs −0.3 ± 1.8 kg, P < 0.001). Decline in BMD at all three sites measured was significantly greater in females compared with males. The average annual change in total hip, femoral neck and lumbar spine BMD was -0.7%, -0.8% and -0.5% in women, and -0.2%, -0.2% and 0.4% in men, respectively. Using the lowest T-score among the three sites, the proportion of participants with low bone density (T-score between -1 to -2.5) and osteoporosis (T-score ≤-2.5) increased significantly in women (baseline 37.8% and 6.3% vs 6 years 49.0% and 10.2%, P < 0.001) and slightly in men (baseline 21.4% and 1.1% vs 6 years 22.7% and 1.2%, P = 0.037).
∆ Total mass
In women, restricted cubic spline analyses showed positive, non-linear relationships between ∆TM and ∆BMD at total hip, femoral neck and lumbar spine with flattening of the curves at ∆TM above +5kg (Fig. 2A). Women in the highest quartile of ∆TM (Q4, with a mid-quartile value of +5.7 kg) had 0.035, 0.018 and 0.025 g/cm2 less reduction in total hip, femoral neck and lumbar spine BMD, respectively, compared with those in the lowest quartile (Q1, with a mid-quartile value of -5.0 kg) (Table 2). In men, ∆TM was positively related to ∆BMD at total hip and femoral neck with no plateau apparent in the relationships; ∆TM was not significantly associated with ∆BMD at the lumbar spine. Men in the highest quartile of ∆TM (Q4, mid-quartile value +5.0kg) had 0.017 and 0.008 g/cm2 less reduction in total hip and femoral neck BMD, respectively, compared with those in the lowest quartile (Q1, mid-quartile value -4.8 kg).
The association of change in total mass (A), lean mass (B) and fat mass (C) with change in bone mineral density (BMD) of total hip, femoral neck and lumbar spine from baseline to follow-up, based on fitted restricted cubic spline regression with three knots (10th, 50th and 90th percentile), adjusted for race, measurement interval between baseline and follow-up, baseline BMD value, age, total mass (or lean and fat mass for the models for ∆lean mass or ∆fat mass), height, smoking history, serum 25-hydroxyvitamin D, physical activity level, sitting hours per day, alcohol consumption, education level, calcium supplementation and menopausal hormone treatment (in females); models for ∆lean mass or ∆fat mass adjusted for each other. Grey shadow represents 95% confidence interval
∆ Lean mass
In females, ∆LM was positively associated with ∆BMD at all three sites measured up to ~1 kg lean mass gain, above which the associations plateaued (Fig. 2B). Women in the highest quartile of ∆LM (Q4, mid-quartile value +1.6 kg) had 0.019-0.028 g/cm2 less bone loss than women in Q1 (mid-quartile value -2.1 kg) (Table 2). In males, ∆LM positively associated with ∆BMD of total hip and femoral neck in near-linear relationships (Fig. 2B), whereas ∆LM was not associated with ∆BMD at the spine. Men in Q4 (mid-quartile value +1.6 kg) had 0.015 and 0.011 g/cm2 less reduction in total hip and femoral neck BMD, respectively, than men in Q1 (mid-quartile value -2.7 kg) (Table 2).
∆ Fat mass
In women, ∆FM was positively associated with ∆BMD of total hip, with plateauing of the relationship at ∆FM of ~+3 kg, whereas ∆FM was not significantly associated with ∆BMD at femoral neck or spine (Fig. 2C). Women in the highest quartile of ∆FM (Q4, mid-quartile value +5.1 kg) had 0.017 g/cm2 less reduction in ∆BMD of total hip compared with women in Q1 (mid-quartile value -3.8 kg). Results were similar in men, with a significant association between ∆FM and ∆BMD of total hip, but not femoral neck or spine. Men in Q4 for ∆FM (mid-quartile value +4.5 kg) had 0.007 g/cm2 less bone loss than men in Q1 (mid-quartile value -3.4 kg) (Table 2).
Analysis by changes in FM and LM
Table 3 shows the analyses where body mass stable participants (774 females and 815 males) were grouped according to whether fat mass and lean mass increased or decreased over time. Group 3 (↑LM & ↓FM) had better maintenance of total hip BMD compared with Group 2 (↓LM & ↑FM) in both sexes, and better maintenance of femoral neck BMD compared with Group 1(↓LM & ↓FM) and Group 2 in males. In females, Group 4 (↑LM & ↑FM) also had better maintenance of total hip BMD compared with Group 1 and Group 2.
Discussion
In this study of a representative cohort of middle to older aged Australians, in females we observed a positive, non-linear association of ∆TM and ∆LM with ∆BMD of total hip, femoral neck and lumber spine, with a threshold level of ~+5kg for ∆TM and ~+1kg for ∆LM. In males a positive, near-linear association of ∆TM and ∆LM with ∆BMD of total hip and femoral neck, but not lumbar spine, was observed. ∆FM was positively associated with ∆total hip BMD in both sexes, with a threshold level of ~3 kg gain in fat mass, but was not significantly associated with ∆BMD of femoral neck or lumbar spine.
Our study is the first longitudinal study to show that maintenance or increase of lean mass is associated with reduction in age-related bone loss in middle to older aged adults, whereas maintenance or increase in fat mass plays a lesser role for total hip BMD and was not associated with changes in femoral neck and lumber spine BMD. Furthermore, in body mass stable participants we showed that those who had increased lean mass but decreased fat mass over time (Group 3) had better maintenance of total hip BMD in both sexes and better maintenance of femoral neck BMD in males compared with those who had decreased lean mass but increased fat mass over time (Group 2). Previous observational studies [22, 23] and weight loss RCTs [24] showed that a decline in body mass was associated with bone loss, but there have been limited data on the relative contributions of change in lean and fat mass. To our knowledge, the only other longitudinal study that evaluated changes in body composition and BMD is The Study of Women’s Health Across the Nation, which focused on the changes in lean and fat mass during the menopause transition period and evaluated lumbar spine and femoral neck BMD [15]. By including both male and female participants, having a larger sample size and wider age range (baseline age 46-70 years), and evaluating total hip BMD which has better precision compared with femoral neck BMD and is the preferred site for monitoring changes in older individuals who tend to have lumbar spine artifact [25], our study advances knowledge on the complicated association between body composition and bone health, and shows the importance of maintaining lean mass for preventing age-related bone loss.
Cross-sectional studies in young, middle-age and older adults have shown positive associations between lean mass and BMD [11, 12, 26]. The important role of lean mass in preserving bone mass is also supported by meta-analysis of RCTs, which showed that exercise could attenuate femoral neck bone loss resulted from diet-induced weight loss [27]. Several mechanisms could link lean body mass with bone mass, including direct mechanical loading (muscle contraction and gravitational loading) [28], genetic factors, hormonal factors, physical inactivity and nutrition factors that related to both muscle and bone loss [29, 30]. In addition, muscle-derived myokines can affect bone remodelling, for example irisin could improve osteoblastgenesis whereas follistatin could inhibit myostatin induced bone resorption [31]. Our study showed that women and men in the highest quartile of ∆LM had 2.8% and 1.4% less loss of total hip BMD over 6 years, respectively, compared with those in the lowest quartile. A 2% improvement in total hip BMD is related to 28% reduction in vertebral fracture risk and 16% reduction in hip fracture risk [32]. Thus, the reduced bone loss observed in our study could be clinically relevant and of importance for fracture prevention.
In cross-sectional studies, associations between fat mass and BMD had been reported to be positive, neutral or negative, depending on participants’ age and sex, as well as analytical methods and covariates adjusted [11,12,13, 26, 33]. In an analysis of 10,814 participants aged 20-59 from NHANES 2011-2018, after accounting for lean mass, fat mass had a moderate, negative association with total body BMD, especially in men [33]. Adipose tissue influences BMD directly through gravitational loading and has indirect, positive effects via endocrine mechanisms, including aromatization of androgens to estrogens, secretion of leptin by adipocytes and increased pancreatic insulin secretion in individuals with higher fat mass [34]. However, adipose tissue can also negatively impact bone through production of inflammatory cytokines which increase bone resorption [35]. Furthermore higher fat mass could negatively affect bone metabolism by reduced circulating 25OHD levels, increased production of parathyroid hormone [36], and dysregulation of the growth hormone /insulin-like growth factor-1 axis [37]. These together could account for the weak or absence of associations with ∆BMD for ∆FM in our study. In the context of the global epidemic of obesity, our findings are of importance in that gain in fat mass provides little benefits in preventing bone loss in older age.
We observed stronger associations between changes in TM, LM and FM with changes in BMD in females compared with males. Partly it could be due to that females had greater bone loss compared with males during the follow up period. The annual rate of change was -0.7% to -0.8% in women and -0.2% in men for total hip/femoral neck BMD, and -0.5% in women and 0.4% in men for lumbar spine BMD, which are comparable to those reported in other longitudinal cohort studies [38, 39]. Another possible explanation is that estrogen receptors have been reported to have different actions on male and female skeleton during loading. In animal studies, estrogen receptor alpha (ERα) plays a critical role in the adaptive response of bone to loading in female mice [40], but not in male mice [41]. Interestingly, an association of change in body mass and bone loss of lumbar spine was only observed in women, which was mainly related to change in lean mass. In males neither change in total mass, nor changes in lean or fat mass associated with change in lumbar spine BMD. The increase in lumbar spine BMD over time observed in males might indicate the high prevalence of spinal osteoarthritis in older men [42], which could influence the accuracy of lumbar spine DXA BMD assessment and obscure any potential association with change in body composition. In addition, previous studies have shown that lumbar spine BMD is less influenced by weight loss compared with total hip BMD [43].
Our study is the first large population-based longitudinal study to evaluate the relationships between change in body composition and BMD during middle to older age that included both male and female participants. In both sexes we showed the importance of maintaining lean mass for preventing age-related bone loss. Assessment at baseline and 6 years allowed us to examine the longitudinal associations of body composition and bone density over sufficient interval to assess age-related bone loss. Our study does have several limitations. Firstly, it is observational in nature and although in the restricted cubic spline analyses we controlled for important confounding variables including lifestyle factors, it remains possible that the greater bone loss observed with greater decline body mass/lean mass was due to uncontrolled or residual confounding, as we were unable to take into account the reason for weight loss and whether it was voluntary or involuntary (i.e. due to medical reasons). Secondly, only those who were able to attend the year 6 survey were included in this study, and therefore our study population might be healthier than people of similar age. Thirdly, DXA technology uses a three-compartment model and assumes that fat free mass has the same hydration. However, it has high reproducibility and has been shown to have strong agreement for body fat % with the reference 4C Lohman model (R2=0.93) [44]. Lastly, we used IPAQ to assess physical activity, which although has been validated for adults [45], does not provide information on activity type. The impact of physical activity type and change in physical activity on changes in body composition and BMD deserves further study. Furthermore, the majority of participants were white, therefore further research in other ethnic groups is needed.
In conclusion, in this longitudinal study of middle-aged and older Australians, loss of body mass is associated with increased bone loss in both males and females. Changes in lean body mass are a stronger determinant than changes in fat mass of changes in bone density. Interventions which preserve or increase lean mass in ageing adults may have long term skeletal benefits.
References
Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17(12):1726–1733
Lloyd JT, Alley DE, Hawkes WG, Hochberg MC, Waldstein SR, Orwig DL (2014) Body mass index is positively associated with bone mineral density in US older adults. Arch Osteoporos 9:175
Beck TJ, Petit MA, Wu G, LeBoff MS, Cauley JA, Chen Z (2009) Does obesity really make the femur stronger? BMD, geometry, and fracture incidence in the women's health initiative-observational study. J Bone Miner Res 24(8):1369–1379
Mortensen SJ, Beeram I, Florance J, Momenzadeh K, Mohamadi A, Rodriguez EK, von Keudell A, Nazarian A (2021) Modifiable lifestyle factors associated with fragility hip fracture: a systematic review and meta-analysis. J Bone Miner Metab 39(5):893–902
Lyall DM, Celis-Morales C, Ward J, Iliodromiti S, Anderson JJ, Gill JMR, Smith DJ, Ntuk UE, Mackay DF, Holmes MV et al (2017) Association of body mass index with cardiometabolic disease in the UK Biobank: a Mendelian Randomization study. JAMA Cardiol 2(8):882–889
Zhu K, Hunter M, James A, Lim EM, Walsh JP (2015) Associations between body mass index, lean and fat body mass and bone mineral density in middle-aged Australians: The Busselton Healthy Ageing Study. Bone 74:146–152
Lloyd JT, Alley DE, Hochberg MC, Waldstein SR, Harris TB, Kritchevsky SB, Schwartz AV, Strotmeyer ES, Womack C, Orwig DL et al (2016) Changes in bone mineral density over time by body mass index in the health ABC study. Osteoporos Int 27(6):2109–2116
Schafer AL (2016) Decline in bone mass during weight loss: a cause for concern? J Bone Miner Res 31(1):36–39
Von Thun NL, Sukumar D, Heymsfield SB, Shapses SA (2014) Does bone loss begin after weight loss ends? Results 2 years after weight loss or regain in postmenopausal women. Menopause 21(5):501–508
Crandall CJ, Yildiz VO, Wactawski-Wende J, Johnson KC, Chen Z, Going SB, Wright NC, Cauley JA (2015) Postmenopausal weight change and incidence of fracture: post hoc findings from Women's Health Initiative Observational Study and Clinical Trials. BMJ 350:h25
Ho-Pham LT, Nguyen UD, Nguyen TV (2014) Association between lean mass, fat mass, and bone mineral density: a meta-analysis. J Clin Endocrinol Metab 99(1):30–38
Zhu K, Briffa K, Smith A, Mountain J, Briggs AM, Lye S, Pennell C, Straker L, Walsh JP (2014) Gender differences in the relationships between lean body mass, fat mass and peak bone mass in young adults. Osteoporos Int 25(5):1563–1570
Reid IR, Ames R, Evans MC, Sharpe S, Gamble G, France JT, Lim TM, Cundy TF (1992) Determinants of total body and regional bone mineral density in normal postmenopausal women--a key role for fat mass. J Clin Endocrinol Metab 75(1):45–51
Kim KM, Lim S, Oh TJ, Moon JH, Choi SH, Lim JY, Kim KW, Park KS, Jang HC (2018) Longitudinal changes in muscle mass and strength, and bone mass in older adults: gender-specific associations between muscle and bone losses. J Gerontol A Biol Sci Med Sci 73(8):1062–1069
Shieh A, Karlamangla AS, Karvonen-Guttierez CA, Greendale GA (2023) Menopause-related changes in body composition are associated with subsequent bone mineral density and fractures: Study of Women's Health Across the Nation. J Bone Miner Res 38(3):395–402
James A, Hunter M, Straker L, Beilby J, Bucks R, Davis T, Eikelboom RH, Hillman D, Hui J, Hung J et al (2013) Rationale, design and methods for a community-based study of clustering and cumulative effects of chronic disease processes and their effects on ageing: the Busselton Healthy Ageing Study. BMC Public Health 13:936
Zhu K, Hunter M, James A, Lim EM, Cooke BR, Walsh JP (2020) Relationship between visceral adipose tissue and bone mineral density in Australian baby boomers. Osteoporos Int 31(12):2439–2448
IPAQ. IPAQ scoring protocol. https://sites.google.com/site/theipaq/scoring-protocol. Accessed Feburary 1, 2022
Harrell FE Jr (2023) R Package ‘rms’. R Foundation for Statistical Computing, Vienna, Austria https://cran.r-project.org/web/packages/rms/rms.pdf
Bredella MA (2017) Sex differences in body composition. Adv Exp Med Biol 1043:9–27
Stevens J, Truesdale KP, McClain JE, Cai J (2006) The definition of weight maintenance. Int J Obes (Lond). 30(3):391–399
Ensrud KE, Ewing SK, Stone KL, Cauley JA, Bowman PJ, Cummings SR (2003) Study of Osteoporotic Fractures Research Group. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. J Am Geriatr Soc 51(12):1740–1747
Ensrud KE, Fullman RL, Barrett-Connor E, Cauley JA, Stefanick ML, Fink HA, Lewis CE, Orwoll E (2005) Osteoporotic Fractures in Men Study Research Group. Voluntary weight reduction in older men increases hip bone loss: the osteoporotic fractures in men study. J Clin Endocrinol Metab 90(4):1998–2004
Villareal DT, Fontana L, Das SK, Redman L, Smith SR, Saltzman E, Bales C, Rochon J, Pieper C, Huang M et al (2016) Effect of two-year caloric restriction on bone metabolism and bone mineral density in non-obese younger adults: a randomized clinical trial. J Bone Miner Res 31(1):40–51
Leslie WD, Ward LM (2004) Bone density monitoring with the total hip site: time for a re-evaluation? J Clin Densitom 7(3):269–274
Leslie WD, Orwoll ES, Nielson CM, Morin SN, Majumdar SR, Johansson H, Oden A, McCloskey EV, Kanis JA (2014) Estimated lean mass and fat mass differentially affect femoral bone density and strength index but are not FRAX independent risk factors for fracture. J Bone Miner Res 29(11):2511–2519
Mesinovic J, Jansons P, Zengin A, de Courten B, Rodriguez AJ, Daly RM, Ebeling PR, Scott D (2021) Exercise attenuates bone mineral density loss during diet-induced weight loss in adults with overweight and obesity: A systematic review and meta-analysis. J Sport Health Sci 10(5):550–559
Kohrt WM, Barry DW, Schwartz RS (2009) Muscle forces or gravity: what predominates mechanical loading on bone? Med Sci Sports Exerc 41(11):2050–2055
Trajanoska K, Rivadeneira F, Kiel DP, Karasik D (2019) Genetics of bone and muscle interactions in humans. Curr Osteoporos Rep 17(2):86–95
Edwards MH, Dennison EM, Aihie Sayer A, Fielding R, Cooper C (2015) Osteoporosis and sarcopenia in older age. Bone 80:126–130
Kirk B, Feehan J, Lombardi G, Duque G (2020) Muscle, bone, and fat crosstalk: the biological role of myokines, osteokines, and adipokines. Curr Osteoporos Rep 18(4):388–400
Bouxsein ML, Eastell R, Lui LY, Wu LA, de Papp AE, Grauer A, Marin F, Cauley JA, Bauer DC, Black DM et al (2019) Change in bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res 34(4):632–642
Jain RK, Vokes T (2022) Fat mass has negative effects on bone, especially in men: a cross-sectional analysis of NHANES 2011-2018. J Clin Endocrinol Metab 107(6):e2545–e2e52
Reid IR (2010) Fat and bone. Arch Biochem Biophys 503(1):20–27
Braun T, Schett G (2012) Pathways for bone loss in inflammatory disease. Curr Osteoporos Rep 10(2):101–108
Bolland MJ, Grey AB, Ames RW, Horne AM, Gamble GD, Reid IR (2006) Fat mass is an important predictor of parathyroid hormone levels in postmenopausal women. Bone 38(3):317–321
Pijl H, Langendonk JG, Burggraaf J, Frolich M, Cohen AF, Veldhuis JD, Meinders AE (2001) Altered neuroregulation of GH secretion in viscerally obese premenopausal women. J Clin Endocrinol Metab 86(11):5509–5515
Berger C, Langsetmo L, Joseph L, Hanley DA, Davison KS, Josse R, Kreiger N, Tenenhouse A, Goltzman D (2008) Canadian Multicentre Osteoporosis Study Research Group. Change in bone mineral density as a function of age in women and men and association with the use of antiresorptive agents. CMAJ 178(13):1660–1668
Moilanen A, Kopra J, Kroger H, Sund R, Rikkonen T, Sirola J (2022) Characteristics of long-term femoral neck bone loss in postmenopausal women: a 25-year follow-up. J Bone Miner Res 37(2):173–178
Lee K, Jessop H, Suswillo R, Zaman G, Lanyon L (2003) Endocrinology: bone adaptation requires oestrogen receptor-alpha. Nature 424(6947):389
Callewaert F, Bakker A, Schrooten J, Van Meerbeek B, Verhoeven G, Boonen S, Vanderschueren D (2010) Androgen receptor disruption increases the osteogenic response to mechanical loading in male mice. J Bone Miner Res 25(1):124–131
Liu G, Peacock M, Eilam O, Dorulla G, Braunstein E, Johnston CC (1997) Effect of osteoarthritis in the lumbar spine and hip on bone mineral density and diagnosis of osteoporosis in elderly men and women. Osteoporos Int 7(6):564–569
Zibellini J, Seimon RV, Lee CM, Gibson AA, Hsu MS, Shapses SA, Nguyen TV, Sainsbury A (2015) Does diet-induced weight loss lead to bone loss in overweight or obese adults? a systematic review and meta-analysis of clinical trials. J Bone Miner Res 30(12):2168–2178
Ng BK, Liu YE, Wang W, Kelly TL, Wilson KE, Schoeller DA, Heymsfield SB, Shepherd JA (2018) Validation of rapid 4-component body composition assessment with the use of dual-energy X-ray absorptiometry and bioelectrical impedance analysis. Am J Clin Nutr 108(4):708–715
Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF et al (2003) International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35(8):1381–1395
Acknowledgments
We thank the operational team in Busselton for participant recruitment and data collection, laboratory staff in Perth for specimen handling, processing and storage, and the community of Busselton for their ongoing support and participation.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Core funding of the Busselton Healthy Ageing Study is provided by the Federal Government of Australia, the Government of Western Australia (Department of Health and Department of Jobs, Tourism, Science and Innovation), the National Health and Medical Research Council (NHMRC) Equipment Grant scheme, the City of Busselton and private donations to the Busselton Population Medical Research Institute. Financial support has also been provided by School of Physiotherapy, Curtin University; Ear Science Institute Australia Inc.; Lions Eye Institute and Lions Hearing Foundation of Western Australia Inc. We thank the Western Australian Country Health Service – South West for core infrastructure support. In-kind equipment and consumable support for the BHAS is gratefully received from Abbott Australasia Pty Ltd, ResMed Science Centre, Stallergens and BD Biosciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Kun Zhu, Michael Hunter, Alan James, Ee Mun Lim, John P Walsh declare no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zhu, K., Hunter, M., James, A. et al. Relationships between longitudinal changes in body composition and bone mineral density in middle-to-older aged Australians. Osteoporos Int 34, 1601–1611 (2023). https://doi.org/10.1007/s00198-023-06773-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-023-06773-z