Abstract
This umbrella review aimed at assessing whether a protein intake exceeding the current recommendation for younger (0.8 g/kg body weight [BW]/day) and older (1.0 g/kg BW/day) adults affects bone mineral density and fracture risk. Moreover, the effect of animal or plant protein was evaluated. A systematic literature search was conducted in PubMed, Embase, and Cochrane Database of Systematic Reviews for systematic reviews (SRs) with or without meta-analysis of prospective studies published between 11/2008 and 08/2021. Methodological quality, outcome-specific certainty of evidence, and overall certainty of evidence of the retrieved SRs were assessed using established tools and predefined criteria. Eleven SRs of randomized controlled trials (RCTs) and/or cohort studies were included. In SRs of cohort studies and RCTs, protein intake/kg BW/day ranged between 0.21–0.95 g (low intake) and > 1.24 g (high intake), respectively, and between 0.67–1.1 g (control groups) and 1.01–1.69 g (intervention groups), respectively. The vast majority of outcome-specific certainty of evidence was rated “low” or “very low.” The overall certainty of evidence for an association (cohort studies) or effect (RCTs) of total, animal or plant protein intake on each of the investigated outcomes was rated “insufficient,” with the exception of possible evidence for a reduced hip fracture risk by high vs. low protein intake. Since protein intakes in low/control and high/intervention groups were very heterogeneous and with low certainty of evidence, it remains unclear whether a dose above the current recommendation or type of protein intake (animal or plant protein) affects bone health overall. However, there is possible evidence for reduced hip fracture risk with high versus low protein intake.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An adequate bone strength is important for proper bone function [1], whereas low bone mineral density (BMD) is associated with an increased risk of osteoporotic fractures [2].
Protein plays an important role in bone health [3] and preservation of muscle mass [4]. Bone and muscle are interconnected tissues whose mass and function are integrated at the biological as well as mechanical level [3, 5]. Muscle forces are the most important stimuli for bone mass maintenance and accretion [6], whereas mechanical unloading results in negative nitrogen balance, loss of muscle mass, and bone catabolism [7].
Mechanistically, dietary proteins provide important components for both bone and skeletal muscle mass which act in different ways: first, they provide amino acids essential for the synthesis of bone matrix and skeletal muscle proteins. Second, specific amino acids such as branched-chained amino acids stimulate gene expression of insulin-like growth factor-1, a hormone that exerts anabolic effects on bone and muscle [8, 9]. Third, whey peptide has been suggested to decrease bone and muscle breakdown during aging through anti-inflammatory pathways [10].
In animal models, both low and high dietary protein intakes have shown to suppress the acquisition of bone mass and the increase in bone strength during growth in comparison to moderate protein intake [11]. In prepubertal boys, a low vs. higher protein intake also impacts weight-bearing peak bone mass and strength [12]. In 2017, the revised reference values for the intake of protein of the nutrition societies of Germany, Austria, and Switzerland (D-A-CH) were set for adults ≤ 65 years and adults > 65 years of age at 0.8 and 1.0 g protein/kg body weight [BW]/day, respectively [13]. A recent review [14] has updated the knowledge about the effects of dietary protein on the risk of osteosarcopenia (the joint loss of bone density and muscle mass). The authors concluded that a protein intake of 1.2–1.5 g/kg BW/day may attenuate osteosarcopenia in older adults (> 65 years of age).
The upcoming evidence-based guideline for protein intake of the German Nutrition Society (DGE) addresses the key questions of whether dietary protein intake with regard to quantitative (higher vs. lower) and qualitative (overall, plant-based, animal-based) considerations affects the development of selected health-related outcomes, among them bone health, in the general adult population. The aim of the present umbrella review was whether a protein intake exceeding the currently recommended protein intake for younger and older adults exerts beneficial or adverse effects on bone metabolism markers, total or site-specific bone mineral density (BMD), and fracture risk. In addition, the effect of the type of protein (animal or plant protein) was evaluated.
Methods
We conducted an umbrella review (PROSPERO: CRD42018082395) following the methodology published by Kroke et al. [15]. All methodological steps were conducted independently by two authors. Any disagreements were resolved by discussion to achieve consensus.
Literature search
A systematic literature search was conducted in PubMed, Embase, and Cochrane Database of Systematic Reviews for sytematic reviews (SRs) published between 1 November 2008 and 17 August 2021. The date of 1 November 2008 originates from the decision to cover a 10-year period; i.e., the initial database search was conducted on 16 November 2018, and the last update was made on 17 August 2021. The search strategy is presented in Supplementary Material S1. In addition to the database search, reference lists of included SRs were screened for further SR of relevance.
Literature selection
Titles and/or abstracts of retrieved studies were screened according to predefined inclusion and exclusion criteria [15] to identify potentially eligible SRs. The full texts of these records were retrieved and assessed for eligibility. It was tolerated that some of the primary studies were incorporated more than once in different SRs. An overview of primary studies included in different SRs is shown in Supplementary Material S2.
Publications were included if they met the following criteria: (i) evaluated the association between protein intake and bone health, (ii) population was the general adult population including older adults and recreational athletes, (iii) study design was an SR with or without meta-analysis (MA) of prospective studies with human study participants, i.e., randomized controlled trials (RCTs), prospective cohort studies, case-cohort studies, or nested case-control studies. SRs also considering case-control studies were only included if prospective studies were predominant (> 50% of all studies), (iv) written in English or German, and (v) published between 11/2008 and 08/2021 [15].
Data extraction
Relevant data from each SR included were extracted into a standardized table.
Approaches to assess the quality and certainty of evidence
To reach a conclusion regarding protein intake and bone health, we proceeded in three steps. First, we assessed the methodological quality of retrieved SRs. Second, we used a scoring tool to assess the certainty of evidence of an association or effect between protein intake and different bone health-related outcomes such as fracture, BMD, and biomarkers of bone metabolism. Third, we rated the overall certainty of evidence separately for each relevant exposure–outcome association (e.g., protein intake and fracture risk) considering all relevant SRs.
Methodological quality of SRs
For quality assessment, a modified version of the AMSTAR 2 (A Measurement Tool to Assess Systematic Reviews 2) tool [16] was used (Supplementary Material S3). This version of AMSTAR 2 contains 14 items evaluating the methodological quality of the SR. SRs were rated on a scale from high quality to critically low quality according to the existence of critical and non-critical methodological weaknesses. SRs rated as “critically low” by AMSTAR 2 were excluded from the rating of the overall certainty of evidence.
Outcome-specific certainty of evidence of SRs
The outcome-specific certainty of evidence of included SRs with and without MA was assessed using a modified version of the NutriGrade scoring tool [17] (Supplementary Materials S4 and S5), and the modifications are described in detail in our methodological protocol [16]. NutriGrade aims to assess the certainty of evidence of an association or effect between different dietary factors and outcomes, taking into account nutrition research-specific requirements not considered by other tools. An important novelty of NutriGrade was the modified classification for MA of RCTs and cohort studies compared with the traditional GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) approach (initially classifying RCTs with a high score and cohort studies with a low score) [15]. This tool utilizes a numerical scoring system and comprises seven items for SRs with MA of RCTs and eight items for MA of cohort studies. Based on a scoring system of a maximum of 10 points, the potential outcome-specific certainty of the evidence was rated based on four categories ranging from high (≥ 8 points) to moderate (6 to < 8 points) to low (4 to < 6 points) to very low (0 to < 4 points). Risk of bias contributes 3 points for RCTs and 2 points for cohort studies to the scale. The NutriGrade scoring tool was modified for the assessment of SRs without MA; the adaptions have already been described in detail by Kroke et al. [15]. For SRs reporting more than one relevant outcome, each outcome was assessed separately.
Rating the overall certainty of evidence and deriving conclusions
The overall certainty of evidence was assessed separately for each relevant exposure-outcome combination according to the framework outlined in the protocol on methodological procedure [15] and in Table 1. Briefly, the overall rating ranges from convincing, probable, possible, to insufficient. At first, we assessed whether there is at least one SR with or without MA of prospective studies. If more than one SR with or without MA was available, all (convincing) or the majority (probable, possible) of the results must be consistent. Biological plausibility must be given in any case (positive or inverse association). In the final step, the results of the AMSTAR 2 and NutriGrade ratings were considered. Depending on the level of evidence, the SRs must have achieved a certain rating in both tools. If no SR is identified or if the majority of SRs reached a very low outcome-specific certainty of evidence and/or a low methodological quality, the overall certainty of evidence was considered insufficient. For this publication, two authors (AZ, HBF) made suggestions for rating the overall certainty of evidence. This rating was double-checked by a staff member of the German Nutrition Society (NK) and thereafter reviewed by all co-authors. The final ratings of the overall certainty of evidence were approved by all authors.
Results
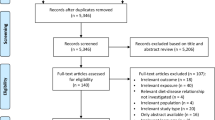
In total, 7336 records were initially identified by literature search. After the removal of duplicates, 6421 records were screened based on title and abstract. We identified 128 potentially eligible records, and eleven SRs were finally considered to be eligible with respect to inclusion and exclusion criteria [18,19,20,21,22,23,24,25,26,27,28]. The literature selection process is outlined in the flow diagram shown in Fig. 1. A list of the excluded studies after full-text assessment, including justifications for exclusion, is provided in Supplementary Material S6. Two SRs were excluded from the evaluation because of a “critically low” AMSTAR 2 rating [29, 30]. The reason for exclusion was that Wallace et al. [29] failed both to conduct an adequate risk of bias assessment and to carry out an adequate investigation of publication bias. Tsagari et al. [30] failed to (i) use a comprehensive literature search strategy, (ii) report the number of excluded studies including their rationale, and (iii) provide an adequate risk of bias assessment.
Characteristics of the included systematic reviews
Table 2 shows the characteristics of the eleven included SRs. These were published between 11/2009 and 01/2020 and considered in total 61 primary studies. Three were SRs with MA of RCTs [21, 23, 27], two were SRs with MA of cohort studies [20, 24], two were SRs of RCTs without MA [18, 22], and one was an SR of cohort studies without MA [26]. One SR with MA [28] and one without MA [25] included both RCTs and cohort studies. A further SR with MA included RCTs, cohort, and cross-sectional studies [19]. The included SRs investigated the following outcomes: fractures [19, 20, 24,25,26, 28], BMD [18,19,20,21,22,23, 25, 27, 28], bone mineral content (BMC) [20, 21], bone metabolism markers [18,19,20, 22, 23, 26], falls [25], and bone loss [25, 26]. Nine SRs investigated the effect/association of total protein intake [19,20,21, 23,24,25,26,27,28], six SRs the effect/association of animal protein intake [18, 19, 24,25,26, 28], five SRs the effect/association of plant protein intake [19, 24,25,26, 28], and one SR the effect of plant vs. animal protein intake [22]. The intervention period of included RCTs ranged from 38 days to 3 years and follow-up of included cohort studies from 1 to 32 years. One SR provided no information on follow-up [24]. One SR was restricted to peri- or postmenopausal women [22], whereas the other ten SRs included data of both sexes. Four SRs focused on older adults [18, 20, 22, 25]. Six SRs were based on adults over 18 years [19, 21, 23, 24, 27, 28] and one on participants aged 14 years or older [26]. In one SR, health status was not reported [24]. The other ten SRs were primarily based on a healthy adult population [18,19,20,21,22,23, 25,26,27,28], but some included additionally studies with subjects suffering from sarcopenia, frailty, overweight, obesity, prehypertension, hypertension, hyperlipidemia, or metabolic syndrome. One SR included exclusively RCTs on participants actively losing weight [21].
Methodological quality
Overall scores of AMSTAR 2 for each included SR are reported in Table 2. Supplementary Material S7 provides a more detailed overview showing the assessments of each individual item. Methodological quality was rated as high for five SRs [18, 20, 24, 25, 27], moderate for two SRs [26, 28], and low for four SRs [19, 21,22,23].
Outcome-specific certainty of the evidence
Overall scores of NutriGrade for each SR are summarized in Table 2. Briefly, out of the 57 NutriGrade ratings, 28 were very low, 24 were low, and five were moderate. Supplementary Material S8 provides a more detailed account showing the assessments of each individual NutriGrade item.
Range and type of protein intake
In SRs of cohort studies (Table 2), the range of protein intake was > 1.2 g/kg BW/day (high protein intake) vs. < 0.21-0.95 g/kg BW/day (low protein intake) or ranged from 17.4 to 30.8 Energy% (En%) vs. 5.6 to 19.9 En%. There was a large overlap of total protein intake in SRs, with high and low intake ranging from > 50.1 g/day vs. < 68.0 g/day, respectively. Similarly, the intake of animal and plant protein showed large overlap, ranging from > 20.6 g/day (high animal protein intake) vs. < 51.0 g/day (low animal protein intake) and from > 13.3 g/day (high plant protein intake) vs. < 18.0 g/day (low plant protein intake), respectively.
In SRs of RCTs (Table 2), protein intake ranged from 1.01 to 1.69 g/kg BW/day (intervention groups) vs. 0.67 to 1.1 g/kg BW/day (control groups) or from 20 to 30 En% vs. 14 to 18 En%. Total protein intake varied largely between single SRs in intervention groups and control groups (20.4–45.0 g protein/day vs. 0–2.1 g protein/day, respectively). The range of animal protein intake was 25 to 30 En% vs. 15 to 18 En% or ranged from 1.2 g/kg BW/day vs. 1.1 g/kg BW/day. In the SRs, the underlying RCTs provided from 40 mg/day to 45 g/day animal protein (intervention groups) vs. 0 to 2.1 g/day (control groups). The range of plant protein was 18 to 40 g/day vs. 0 g/day. One SR with MA [22] compared animal protein vs. plant protein with supplement doses ranged from 18 to 40 g/day vs. placebo. Although protein intake between the included SRs varied strongly, even within groups of high and low intakes (cohort studies) or intervention and control groups (RCTs), we tried to answer our research question by comparing high vs. low protein intake and intervention vs. control groups.
Fracture risk
All SRs regarding fracture risk were exclusively based on data from observational studies. Four SRs with MA [19, 20, 24, 28] and two SRs without MA [25, 26] reported data on protein intake and total fracture risk. The vast majority of SRs did not observe an association of high protein intake vs. low protein intake on total fracture risk, neither for total protein intake (three out of four SRs) nor for plant protein intake (three out of three SRs) [19, 24, 26]. Two SRs [19, 24] observed no association between high vs. low animal protein intake on total fracture risk, whereas two SRs [25, 26] observed a positive association. The two SRs without MA that reported an association between total fracture risk and higher animal protein intake were of high [25] and moderate [26] methodological quality; however, both of them included a single cohort study.
Whereas the methodological quality of the majority of SRs reached at least a “moderate” rating, the majority of SRs reached only a “very low” outcome-specific certainty of evidence. Therefore, the overall certainty of evidence for an association of higher total or plant protein intake on total fracture risk was rated “insufficient.” For the association between animal protein intake and total fracture risk, the overall certainty of evidence was also rated “insufficient” due to inconsistent risk associations.
With respect to hip fracture risk, three SRs with MA were available [20, 24, 28]. Two of them observed an inverse association between higher protein intake and hip fracture risk [20, 24]. Both SRs were of high methodological quality and were based on a higher number of individual studies than the SR by Darling et al. [28] that did not observe an association. Groenendijk et al. [20] reported a significant mean risk reduction of 11% for a total protein intake above 0.93 g/kg BW/day compared to a lower intake in adults aged 65 and older. The SR by Wu et al. [24] included six cohort studies with 270,011 participants (aged 18 to 89 years) and also reported a risk reduction of 11% by high vs. low protein intake. In addition, Wu et al. explored a possible dose-response relationship between the amount of daily protein intake within a daily range of 45 to 105 g protein and hip fracture risk, using data of three sub-studies which met dose-response meta-analysis criteria. Although statistically non-significant, results were generally consistent with their data on low vs. high protein intake. Neither higher intakes of plant nor of animal protein were associated with hip fracture risk in two SRs [24, 28]. Altogether, two out of three SRs reported consistently an inverse association between total protein intake and the risk of hip fractures. The majority of SRs reached a high methodological quality, but were only rated “low” by NutriGrade. Thus, there is possible evidence for a decrease in hip fracture risk for higher vs. lower total protein intake. None of the SRs observed an association between animal or plant protein intake and hip fracture risk. The methodological quality of all SRs reached at least “moderate,” but the outcome-specific certainty of evidence was rated as very low for each SR. Consequently, the overall certainty of evidence was rated “insufficient” for a significant association of higher animal or plant protein intake on hip fracture risk.
One SR with MA reported data on protein intake and limb fracture risk in two cohort studies [24]. Wu et al. [24] did not observe a statistically significant association between total and plant protein intake and limb fracture risk, but there was a strong heterogeneity, and the outcome-specific certainties of the evidence were rated “very low.” Due to the “very low” NutriGrade-rating, the overall certainty of evidence for an absent association between total and plant intake and limb fracture risk was judged to be insufficient.
Bone mineral density
Five SRs with MA [19, 21, 23, 27, 28] and four SRs without MA [18, 20, 22, 25] reported data on BMD at different skeletal sites (Table 2). None of the SRs that examined the relations between total protein intake and total body BMD [20, 21, 25, 27] or total hip BMD [20, 21, 23, 27], respectively, found an association (cohort studies) [20] or effect (intervention studies) [21, 25, 27]. The methodological quality of included SRs was only “moderate,” and the majority of included SRs reached a “low” outcome-specific certainty of evidence. Therefore, the overall certainty of evidence that higher total protein intake does not affect total body/hip BMD was rated as “possible.” For the relationship between total protein intake and total hip BMD, the majority of included SRs reached at least a “low” outcome-specific certainty of evidence, but half of them were of low methodological quality. Accordingly, the overall certainty of evidence was judged as “insufficient” for an absence of an association (cohort studies) or effect (intervention studies) between total protein intake and total body/hip BMD.
Regarding lumbar spine BMD, three out of six SRs including in total seven RCTs reported null effects of higher protein intake [19, 27, 28]. One SR of cohorts did not show any consistent results [20], and the other two SRs reported a statistically significant higher lumbar spine BMD by a higher protein intake [21, 23]. Wright et al. included four RCTs with a total of 322 participants comparing high protein diet (≥ 1 g/kg BW/day) vs. normal protein diet [21]. Lumbar spine BMD was statistically significant and consistently, yet modest, increased by high protein diet. The SR by Shams-White et al. summarized the effect of high vs. low protein intake (> 90 g/day vs. < 80 g/day or 25 to 30 En% vs. 15 to 18 En%) in five RCTs with 989 participants (aged ≥ 18 years) [23]. Higher protein intake statistically significantly increased lumbar spine BMD without evidence for heterogeneity. For both SRs, methodological quality was assessed “low,” and the outcome-specific certainties of evidence were rated as “low.”
Due to the lack of consistent results, the overall certainty of evidence for a relationship between the amount of protein intake and lumbar spine BMD was judged as “insufficient.”
With respect to femoral neck BMD, the vast majority of SRs (three out of four) reported null effects of high vs. low total protein intake with “low” or “very low” outcome-specific certainties of evidence [19, 21, 23]. One SR of six cohort studies did not observe consistent results [20].
Whereas the majority of included SRs reached at least a “low” outcome-specific certainty of evidence, the majority of SRs failed to reach at least a methodological quality of “moderate.” Therefore, the overall certainty of evidence was judged as “insufficient” that higher total protein intake affects femoral neck BMD.
Regarding specific protein sources, Blair et al. [18] investigated the effect of whey protein on BMD in two RCTs with 148 healthy participants (mean age: 50–70.5 years). Both RCTs reported unchanged BMD for supplement doses of 40 mg/day to 40 g/day with an outcome-specific certainty of evidence rated as “low.” Another SR that examined specific protein types found no statistically significant effects of milk or soy protein on lumbar spine BMD or of soy protein on femoral neck BMD [19].
The SR by Shams-White et al. [22] compared the effect of soy vs. animal protein (supplement dose: 18–40 g/day) on BMD at different skeletal sites in peri- or postmenopausal women. None of the included RCTs found statistically significant differences between both protein sources in the net changes in lumbar spine, femoral neck, or total body BMD. The outcome-specific certainties of evidence were “moderate.”
Bone mineral content
There was one SR with MA of RCTs [21] and one SR without MA of cohort studies [20] on protein intake and total body BMC. Groenendijk et al. [20] included two cohort studies with 1406 participants. One cohort study observed no association of high vs. low total protein intake with total body BMC, whereas the other cohort study observed a positive association. The SR by Wright et al. [21] of seven RCTs with 408 participants did not find a statistically significant effect of high protein diet (≥ 1 g/kg BW/day) on total body BMC, or on femoral neck or lumbar spine BMC, the latter two results were based on two RCTs.
The majority of included SRs reached neither a moderate methodological quality nor a low certainty of evidence. Consequently, the overall certainty of evidence was rated “insufficient” that a high total protein intake affects total body BMC. Due to the low methodological quality, the overall certainty of evidence that the amount of protein intake does influence femoral neck and lumbar spine BMC was considered to be insufficient.
Biomarkers of bone metabolism
Markers of bone formation, such as serum osteocalcin and bone-specific alkaline phosphatase (BAP), as well as markers of bone resorption, such as N-terminal telopeptide (NTX) and C-terminal telopeptide (CTX), were investigated in five SRs of RCTs, two of them with MA [19, 23] and three without MA [18, 20, 22].
The SR by Shams-White et al. did not demonstrate a statistically significant effect of total protein supplementation on serum osteocalcin [23]. Furthermore, both cohort studies included in the SR by Groenendijk et al. showed no association between total protein intake and osteocalcin [20]. Blair et al. [18] investigated the effect of whey protein supplementation on osteocalcin, but in two RCTs included, osteocalcin remained unchanged with an outcome-specific certainty of evidence rating as “low.”
Changes in BAP were investigated in two SRs [19, 22]. Darling et al. did not detect a statistically significant effect of soy protein on BAP (mean difference: − 1.75; 95% CI: − 10.50, 7.01) by consideration of six RCTs with in total 128 participants [19]. The SR by Shams-White et al. compared the effect of soy vs. animal protein on BAP in peri- and postmenopausal women, but did not find statistically significant treatment effects [22].
Furthermore, Shams-White et al. examined the effect of soy vs. animal protein on NTX (two RCTs with 91 participants) [22]. There was no statistically significant treatment effect on NTX, and the certainty of evidence was rated “low.” The bone resorption marker CTX was investigated in a single SR [23], which did not find a statistically significant effect of total protein supplementation on CTX. The NutriGrade-rating was “low” [23].
Due to the low methodological quality of the SR by Shams-White et al. [23], the overall certainty of evidence that total protein supplementation does not affect serum osteocalcin or CTX was rated “insufficient.”
With “possible” overall certainty of evidence, there is no association between total protein intake and NTX. The reason for this rating was the high methodological quality of the SR by Groenendijk et al. [20] and the “low” outcome-specific evidence.
Other outcome parameters
Single SRs without MA reported data on bone loss [25, 26] or falls [25]. None of the two SRs found consistent results on the relationship between protein intake and bone loss, either for total [25, 26], animal [25], or plant protein [25]. Pedersen et al. included a single cohort study of 807 older adults to examine the association between protein intake and risk of falls [25]. This cohort study did not report any statistically significant associations between total, animal, or plant protein and the risk of falls. The overall certainty of evidence that there is no relationship between total protein intake and bone loss was judged as “possible” as both SRs included reached at least a methodological quality of “moderate” and the outcome-specific certainty of evidence was “low” for both associations. With regard to animal and plant protein, overall certainty of evidence was rated as possible that their amount does not affect bone loss, because both outcome-specific certainties of evidence were rated as “low” and the methodological quality of the SR by Pedersen et al. [25] was assessed “high.”
Discussion
This umbrella review summarizes the results of several SRs on various parameters of bone health such as biomarkers of bone metabolism, total and site-specific BMD, and fracture risk. The vast majority of outcome-specific certainty of evidence was rated “low” or even “very low.” For most outcome markers, the overall specific certainty of evidence was rated “insufficient,” with the exceptions of “possible” for a reduced hip fracture risk by high versus low protein intake and for no effect of higher protein intake on total BMD (Supplementary Material S9). To the best of our knowledge, this umbrella review is the first to provide a summary evidence assessment of previous SRs.
Osteoporotic fractures are the most important outcomes of impaired bone metabolism. Our results indicate that a beneficial effect of a protein intake above the recommendation (1.0 g/kg BW/day) in adults > 65 years of age on bone health, specifically hip fracture risk, cannot be excluded. The SRs on hip fracture risk included a substantial percentage of elderly people, an age group that is known for an exponential increase in the risk of fractures [31], particularly in nursing home residents [32]. As a higher protein intake may have beneficial effects on skeletal muscle [14], we cannot exclude the possibility that the beneficial effect of a higher protein intake on hip fracture risk reduction supported by three SRs with MA identified here may be explained by beneficial effects on skeletal muscle [33]. The results on hip fracture risk obtained from SRs of observational studies are in line with the results of a secondary prevention trial in older patients with recent osteoporotic hip fracture [34]. This study could demonstrate that a daily protein supplementation of 20 g vs. an isoenergetic placebo attenuates proximal femur bone loss and reduces in-hospital stay in rehabilitation care facilities. At baseline, the protein-supplemented group of that RCT had a daily protein intake of 45 g on average, corresponding to 0.74 g/kg BW/day, which increased by supplementation on average to 1.06 g/kg BW/day. In this context, it is notable that in community-dwelling older adults, the prevalence of a protein intake below 0.8 g/kg BW/day is substantial (14–30%) and increases to 65–76% if a cut-off value of 1.2 g/kg BW/day is considered [35, 36]. The situation seems to be even worse in nursing home residents, where a mean daily protein intake of only 0.7 g/kg BW has been reported [37]. Thus, the high risk of hip fractures in older adults, and particularly in nursing home residents, may, at least in part, be increased by a protein intake below the current recommendation. Since guidelines from expert consensus groups, such as the European Society on Parenteral and Enteral Nutrition (ESPEN), already advocate a higher intake of protein than currently recommended (1.0–1.5 g/kg/day) to retard age-related muscle loss [38], it is possible that the fracture risk in older adults may additionally be attenuated by protein intake > 1.0 g /kg BW/day.
Generally, the evaluation of the effect of protein intake on the risk of fractures is challenging for several reasons: First, it may take years or even decades until a nutrition-related fracture occurs, but it is nearly impossible to perform long-term RCTs regarding the effect of different intakes of a macronutrient like protein on bone health. This explains why only data of observational studies are available regarding protein intake and fracture risk, where under- and overreporting of specific foods has to be considered as this may affect dose-response analysis on protein intake and fracture risk. Second, there may be interactions between protein intake, calcium intake, and physical activity [3, 39, 40], and protein-rich foods, such as meat, milk, or soy, contain many other nutrients, which makes it difficult to separate a potential protein-related effect from the effect of other nutrients. Third, even multivariable-adjusted prospective cohort studies may be biased by unexplained confounding factors not related to nutrition. Finally, low-trauma fractures, which are typical in osteoporotic individuals, are rarely seen in young and middle-aged adults, who were important target populations of this umbrella review.
SRs on BMD are at the interface between studies on fracture as outcome and studies on bone turnover markers, since BMD is linked to bone strength [1] and thus to fracture risk [41]. Studies on BMD have the advantage that substantial effects can be demonstrated already after several months or 1 or 2 years, making even RCTs possible. Nevertheless, evidence from available SRs for an effect of the amount of protein intake on BMD remains insufficient.
Results on biochemical parameters of bone formation and resorption reflect short- to mid-term bone health. Theoretically, dietary protein may have anabolic effects on skeletal muscle or bone protein synthesis, but it may also adversely increase bone resorption by its calciuretic effect [42, 43], particularly if animal protein with its relatively high content of sulfur-containing amino acids is ingested. With respect to the type of protein intake, a large prospective study in 1035 elderly women showed that a higher intake of animal vs. plant protein was associated with a more rapid femoral neck bone loss and a higher risk of hip fracture [44]. According to the acid-base hypothesis, skeletal salts are mobilized from bone to balance acids endogenously generated from sulfur-containing, acid-forming amino acid, which are more prevalent in animal than in plant protein [45]. However, this hypothesis was challenged by the results of a recently published RCT, demonstrating an increased bone turnover among healthy adults by partial replacement of animal by plant protein [46]. Our umbrella review does neither reveal beneficial nor adverse effects on bone turnover markers by protein supplementation. In this regard, soy and animal protein did not differ substantially. In line with these findings, some have argued that the calciuretic effect of protein may be compensated by increased intestinal calcium absorption rather than bone loss [42, 43]. Nevertheless, the results of our umbrella review should be regarded as preliminary, since in the overall certainty of evidence was rated “insufficient.”
We need to point out that the quality of SRs available to date has been limited, especially at the RCT level. Particularly, the quality of the SRs with MA on protein intake and BMD was only low to very low [22, 28], with the exception of the SR with MA on high protein weight loss diets [21], which was of moderate quality. A further major limitation is that most SRs with MA were not restricted to specific risk groups, such as older adults whose risk of fracture and of inadequate energy and protein intake is high, and whose requirement on daily protein intake is probably higher than currently assumed. Therefore, it is important to mention that one of the most recent SR with MA [20] showed that in adults aged ≥ 65, dietary protein intake above 0.93 g/kg BW/day was inversely associated hip fracture risk. In addition, there was a wide and overlapping range of protein intake between groups with low and high protein intakes in different SRs and its underlying cohorts or RCTs, thus hampering the detection of clear dose-response relationships. Finally, it may be not clear why a classical GRADE assessment (instead of NutriGrade) was not performed. We are aware that in the meantime, the GRADE approach was amended in a way that cohort studies can now also be assigned an initially high score, when risk of bias tools such as ROBINS-I are used [47]. However, the adjustments were not published until 2019, whereas the guideline methodology for our umbrella review was established in 2017.
Conclusion
Overall, available data regarding the impact of protein intake on bone health from SRs are insufficient to draw reliable conclusions for the general adult population. However, there is “possible” evidence for a reduced hip fracture risk by high versus low protein intake. Since osteoporotic fractures increase exponentially with higher age [31], and guidelines from expert consensus groups, such as the European Society on Parenteral and Enteral Nutrition (ESPEN), already advocate a higher intake of protein than currently recommended (1.0–1.5 g/kg/day) to retard age-related muscle loss [38], future research and analyses should focus on the effect of higher protein intake on bone health in adults aged 65 or older. In addition, more high-quality research regarding the effect of dose and type of protein on bone health in the entire adult population is needed.
Data availability
Not applicable
Code availability
Not applicable
Abbreviations
- AMSTAR 2:
-
A Measurement Tool to Assess Systematic Reviews 2
- BMD:
-
Bone mineral density
- BW:
-
Body weight
- En%:
-
Energy percentage
- MA:
-
Meta-analysis
- RCT:
-
Randomized controlled trial
- SR:
-
Systematic review
References
Turner CH (2006) Bone strength: current concepts. Ann N Y Acad Sci 1068:429–446. https://doi.org/10.1196/annals.1346.039
Hillier TA, Stone KL, Bauer DC et al (2007) Evaluating the value of repeat bone mineral density measurement and prediction of fractures in older women: the study of osteoporotic fractures. Arch Intern Med 167:155–160. https://doi.org/10.1001/archinte.167.2.155
Chevalley T, Bonjour JP, Ferrari S et al (2008) High-protein intake enhances the positive impact of physical activity on BMC in prepubertal boys. J Bone Miner Res 23:131–142. https://doi.org/10.1359/jbmr.070907
Olaniyan ET, O'Halloran F, McCarthy AL (2021) Dietary protein considerations for muscle protein synthesis and muscle mass preservation in older adults. Nutr Res Rev 34:147–157. https://doi.org/10.1017/S0954422420000219
Bischoff-Ferrari HA (2018) Chapter 68: Prevention of falls. In: Bilezikian JP, Bouillon R, Clemens T et al (eds) Primer on the metabolic bone diseases and disorders of mineral metabolism. Wiley, pp 526–533
Schiessl H, Frost HM, Jee WS (1998) Estrogen and bone-muscle strength and mass relationships. Bone 22:1–6
Scheld K, Zittermann A, Heer M et al (2001) Nitrogen metabolism and bone metabolism markers in healthy adults during 16 weeks of bed rest. Clin Chem 47:1688–1695
Bonjour J-P (2016) The dietary protein, IGF-I, skeletal health axis. Horm Mol Biol Clin Investig 28:39–53. https://doi.org/10.1515/hmbci-2016-0003
Bonjour JP, Ammann P, Chevalley T et al (2001) Protein intake and bone growth. Can J Appl Physiol 26(Suppl):S153–S166. https://doi.org/10.1139/h2001-050
Sugawara K, Takahashi H, Kashiwagura T et al (2012) Effect of anti-inflammatory supplementation with whey peptide and exercise therapy in patients with COPD. Respir Med 106:1526–1534. https://doi.org/10.1016/j.rmed.2012.07.001
Takeda S, Kobayashi Y, Park J-H et al (2012) Effect of different intake levels of dietary protein and physical exercise on bone mineral density and bone strength in growing male rats. J Nutr Sci Vitaminol 58:240–246. https://doi.org/10.3177/jnsv.58.240
Chevalley T, Bonjour J-P, Audet M-C et al (2017) Prepubertal impact of protein intake and physical activity on weight-bearing peak bone mass and strength in males. J Clin Endocrinol Metab 102:157–166. https://doi.org/10.1210/jc.2016-2449
Richter M, Baerlocher K, Bauer JM et al (2019) Revised reference values for the intake of protein. Ann Nutr Metab 74:242–250. https://doi.org/10.1159/000499374
Kirk B, Prokopidis K, Duque G (2021) Nutrients to mitigate osteosarcopenia: the role of protein, vitamin D and calcium. Curr Opin Clin Nutr Metab Care 24:25–32. https://doi.org/10.1097/MCO.0000000000000711
Kroke A, Schmidt A, Amini AM et al (2022) Dietary protein intake and health-related outcomes: a methodological protocol for the evidence evaluation and the outline of an evidence to decision framework underlying the evidence-based guideline of the German Nutrition Society. Eur J Nutr 61:2091–2101. https://doi.org/10.1007/s00394-021-02789-5
Shea BJ, Reeves BC, Wells G et al (2017) AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 358:j4008. https://doi.org/10.1136/bmj.j4008
Schwingshackl L, Knüppel S, Schwedhelm C et al (2016) Perspective: NutriGrade: a scoring system to assess and judge the meta-evidence of randomized controlled trials and cohort studies in nutrition research. Adv Nutr 7:994–1004. https://doi.org/10.3945/an.116.013052
Blair M, Kellow NJ, Dordevic AL et al (2020) Health benefits of whey or colostrum supplementation in adults ≥35 years; a systematic review. Nutrients 12:299. https://doi.org/10.3390/nu12020299
Darling AL, Manders RJF, Sahni S et al (2019) Dietary protein and bone health across the life-course: an updated systematic review and meta-analysis over 40 years. Osteoporos Int 30:741–761. https://doi.org/10.1007/s00198-019-04933-8
Groenendijk I, den Boeft L, van Loon LJ et al (2019) High versus low dietary protein intake and bone health in older adults: a systematic review and meta-analysis. Comput Struct Biotechnol J 17:1101–1112. https://doi.org/10.1016/j.csbj.2019.07.005
Wright CS, Li J, Campbell WW (2019) Effects of dietary protein quantity on bone quantity following weight loss: a systematic review and meta-analysis. Adv Nutr 10:1089–1107. https://doi.org/10.1093/advances/nmz058
Shams-White MM, Chung M, Fu Z et al (2018) Animal versus plant protein and adult bone health: a systematic review and meta-analysis from the National Osteoporosis Foundation. PLoS One 13:e0192459. https://doi.org/10.1371/journal.pone.0192459
Shams-White MM, Chung M, Du M et al (2017) Dietary protein and bone health: a systematic review and meta-analysis from the National Osteoporosis Foundation. Am J Clin Nutr 105:1528–1543. https://doi.org/10.3945/ajcn.116.145110
Wu A-M, Sun X-L, Lv Q-B et al (2015) The relationship between dietary protein consumption and risk of fracture: a subgroup and dose-response meta-analysis of prospective cohort studies. Sci Rep 5:9151. https://doi.org/10.1038/srep09151
Pedersen AN, Cederholm T (2014) Health effects of protein intake in healthy elderly populations: a systematic literature review. Food Nutr Res 58. https://doi.org/10.3402/fnr.v58.23364
Pedersen AN, Kondrup J, Børsheim E (2013) Health effects of protein intake in healthy adults: a systematic literature review. Food Nutr Res 57. https://doi.org/10.3402/fnr.v57i0.21245
Santesso N, Akl EA, Bianchi M et al (2012) Effects of higher- versus lower-protein diets on health outcomes: a systematic review and meta-analysis. Eur J Clin Nutr 66:780–788. https://doi.org/10.1038/ejcn.2012.37
Darling AL, Millward DJ, Torgerson DJ et al (2009) Dietary protein and bone health: a systematic review and meta-analysis. Am J Clin Nutr 90:1674–1692. https://doi.org/10.3945/ajcn.2009.27799
Wallace TC, Frankenfeld CL (2017) Dietary protein intake above the current RDA and bone health: a systematic review and meta-analysis. J Am Coll Nutr 36:481–496. https://doi.org/10.1080/07315724.2017.1322924
Tsagari A (2020) Dietary protein intake and bone health. J Frailty Sarcopenia Falls 5:1–5. https://doi.org/10.22540/JFSF-05-001
Hadji P, Klein S, Gothe H et al (2013) The epidemiology of osteoporosis--bone evaluation study (BEST): an analysis of routine health insurance data. Dtsch Arztebl Int 110:52–57. https://doi.org/10.3238/arztebl.2013.0052
Sawka AM, Ismaila N, Cranney A et al (2010) A scoping review of strategies for the prevention of hip fracture in elderly nursing home residents. PLoS One 5:e9515. https://doi.org/10.1371/journal.pone.0009515
Sahni S, Cupples LA, McLean R et al (2010) Protective effect of high protein and calcium intake on the risk of hip fracture in the Framingham offspring cohort. J Bone Miner Res, Wiley, New Jersey, USA
Schürch MA, Rizzoli R, Slosman D et al (1998) Protein supplements increase serum insulin-like growth factor-I levels and attenuate proximal femur bone loss in patients with recent hip fracture. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 128:801–809. https://doi.org/10.7326/0003-4819-128-10-199805150-00002
Hengeveld LM, Boer JMA, Gaudreau P et al (2020) Prevalence of protein intake below recommended in community-dwelling older adults: a meta-analysis across cohorts from the PROMISS consortium. J Cachexia Sarcopenia Muscle 11:1212–1222. https://doi.org/10.1002/jcsm.12580
de Groot LC, Hautvast JG, van Staveren WA (1992) Nutrition and health of elderly people in Europe: the EURONUT-SENECA study. Nut Rev 50:185–194. https://doi.org/10.1111/j.1753-4887.1992.tb01323.x
Seemer J, Volkert D, Fleckenstein-Sußmann D et al (2021) Usual protein intake amount and sources of nursing home residents with (risk of) malnutrition and effects of an individualized nutritional intervention: an enable study. Nutrients 13:2168. https://doi.org/10.3390/nu13072168
Deutz NE, Bauer JM, Barazzoni R et al (2014) Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr 33:929–936. https://doi.org/10.1016/j.clnu.2014.04.007
Mendonça N, Kingston A, Granic A et al (2020) Contribution of protein intake and its interaction with physical activity to transitions between disability states and to death in very old adults: The Newcastle 85+ Study. Eur J Nutr 59:1909–1918. https://doi.org/10.1007/s00394-019-02041-1
Dawson-Hughes B (2003) Interaction of dietary calcium and protein in bone health in humans. J Nutr 133:852S–854S. https://doi.org/10.1093/jn/133.3.852S
Raisz LG (2005) Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest 115:3318–3325. https://doi.org/10.1172/JCI27071
Bonjour J-P (2011) Protein intake and bone health. Int J Vitam Nutr Res 81:134–142. https://doi.org/10.1024/0300-9831/a000063
Thorpe MP, Evans EM (2011) Dietary protein and bone health: harmonizing conflicting theories. Nutr Rev 69:215–230
Sellmeyer DE, Stone KL, Sebastian A et al (2001) A high ratio of dietary animal to vegetable protein increases the rate of bone loss and the risk of fracture in postmenopausal women. Study of Osteoporotic Fractures Research Group. Am J Clin Nutr 73:118–122
Weaver CM (2021) Plant protein meal patterns may compromise bone health. J Nutr 151:7–8. https://doi.org/10.1093/jn/nxaa346
Itkonen ST, Päivärinta E, Pellinen T et al (2021) Partial replacement of animal proteins with plant proteins for 12 weeks accelerates bone turnover among healthy adults: a randomized clinical trial. J Nutr 151:11–19. https://doi.org/10.1093/jn/nxaa264
Schünemann HJ, Cuello C, Akl EA et al (2019) GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. J Clin Epidemiol 111:105–114. https://doi.org/10.1016/j.jclinepi.2018.01.012
Acknowledgements
We would like to thank all panel members of the guideline on protein intake for their contributions to the methodological approach and specifically to the present manuscript. The following scientists deserve thanks for providing helpful remarks during guideline panel meetings and previous versions of this manuscript: Anna M. Amini, Jürgen M. Bauer, Heiner Boeing, Anette E. Buyken, Anja Carlsohn, Tilman Kühn, Katharina Nimptsch, and Thomas Remer.
Funding
This research was partly funded by the German Federal Ministry of Food and Agriculture. The funder had no role in the decisions regarding data collection, analyses, interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Author information
Authors and Affiliations
Consortia
Contributions
AS, JH, NK, and AL conducted the systematic literature search, literature selection, data extraction, and AMSTAR 2 and NutriGrade evaluations. AZ and HBF evaluated the evidence graded the overall certainty of evidence, which was finalized after the discussion with all guideline panel members. AZ, HBF, AS, and NK prepared the manuscript. All authors read, provided critical input, and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
A list of possible conflicts of interest is provided in Supplementary Material S10. Briefly, Heike Bischoff-Ferrari has received honorariums from WILD Pharma, DSM Nutritional Products, Fresenius, Roche, Meda, Sanofi, financial contributions from Schweizerischer Nationalfond, fundings from Pfizer, Vifor, Wild, Streuli Pharma, and medication from Omanda. Sarah Egert is member of the professional association of nutritionists (VDOE), German Nutrition Society (DGE), German Society for Nutritional Medicine (DGEM), and European Society for Clinical Nutrition and Metabolism (ESPEN). Sabine Ellinger has received various speaker honorariums and is member of the German Nutrition Society. Anja Kroke received financial contributions from technical health insurance (TK), owns shares from SATINA, has personal relationships to the company SATINA, and is member of the German Society for Epidemiology (DGEpi), medical chamber Hessen, and German Nutrition Society (DGE). Sandrine Louis owns shares from OP Food. Stefan Lorkowski is consultant for Akcea Therapeutics, AMGEN, Daiichi Sankyo, Danone, Sanofi-Aventis, Thüringer Ernährungsnetzwerk, has received honorarium for lecturing and training activities (Akcea Therapeutics, amedes, AMGEN, Berlin-Chemie, Boehringer Ingelheim Pharma, Bund Niedergelassener Kardiologen, Daiichi Sankyo, MSD Sharp & Dohme, Novo Nordisk Pharma, Omnimed, Roche Pharma, Sanofi Aventis, Synlab, Unilever, Upfield), is member of the German Nutrition Society (DGE), German Federal Institute for Risk Assessment (BfR), Federation of European Nutrition Societies (FENS), and participant in research projects for the development of reformulated food and participation in projects for improving food education. Matthias Schulze is member of the European Association for the Study of Diabetes (EASD) and German Society for Epidemiology (DGEpi). Lukas Schwingshackl is member of the GRADE Working Group. Roswitha Siener is member of the German Society for Urology (DGU) and German Academy of Nutritional Medicine (DAEM). Dorothee Volkert is member of the advisory board of the company Apetito, has received honorariums from Fresenius, Nestle, Nutricia, research funding from Nestec Ltd., and is member of the German Society for Nutritional Medicine (DGEM) German Nutrition Society, European Society for Clinical Nutrition and Metabolism (ESPEN). Bernhard Watzl is President of the German Nutrition Society (DGE). Gabriele Stangl, Armin Zittermann, Nicole Kalotai, Annemarie Schmidt, Julia Haardt, and Andreas Lehmann declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zittermann, A., Schmidt, A., Haardt, J. et al. Protein intake and bone health: an umbrella review of systematic reviews for the evidence-based guideline of the German Nutrition Society. Osteoporos Int 34, 1335–1353 (2023). https://doi.org/10.1007/s00198-023-06709-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-023-06709-7