Abstract
Summary
From the perspective of Malaysian health care providers, denosumab was cost-effective in the treatment of postmenopausal osteoporosis, with an optimal outcome starting at age 60 years. Our results provide important insights into the value for money of anti-osteoporotic agents that can serve as a reference for other countries with comparable epidemiological data.
Introduction
The study aimed to compare the cost-effectiveness of denosumab with alendronate and no treatment in the management of postmenopausal osteoporosis among the Malaysian population.
Methods
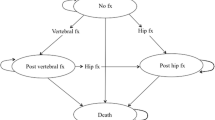
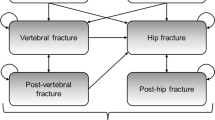
A well-validated Markov model was used to estimate the cost-effectiveness of denosumab in a hypothetical cohort of postmenopausal osteoporotic women between 50 and 80 years old who had no history of fractures. A 10-year time horizon from the perspective of Malaysian health care providers was used in this analysis. The model parameters, including transition probabilities and costs, were based on Malaysian sources. Treatment efficacy data were obtained from a network meta-analysis. The study outcomes were presented as incremental cost per quality-adjusted life-year (QALY) gained. Sensitivity analyses were performed to ensure the robustness of the results. A cost-effectiveness threshold was set at MYR 21,438 (USD 5175) per QALY.
Results
Denosumab was found to be a cost-effective option for postmenopausal osteoporotic women aged 60 and older. The incremental cost-effectiveness ratios (ICERs) for denosumab versus alendronate ranged from MYR 16,955 (USD 4093) per QALY at age 60 to MYR 4380 (USD 1057) per QALY at age 80. The cost-effectiveness of denosumab improved monotonically with increasing age. Denosumab was 72.8–92.7% likely to be cost-effective at the cost-effectiveness threshold. Sensitivity analyses demonstrated that the results were robust across all parameter variations, with the annual cost of denosumab being the most sensitive.
Conclusions
From the perspective of the Malaysian health care provider, denosumab appears to be a cost-effective treatment choice for postmenopausal osteoporotic women over 60 years of age.




Similar content being viewed by others
References
Odén A, McCloskey EV, Kanis JA et al (2015) Burden of high fracture probability worldwide: secular increases 2010–2040. Osteoporos Int 26:2243–2248. https://doi.org/10.1007/s00198-015-3154-6
Department of Statistics Malaysia (2016) Population Projection (Revised), Malaysia, 2010–2040. Malaysia
Cheung C-L, Bin AS, Chadha M et al (2018) An updated hip fracture projection in Asia: The Asian Federation of Osteoporosis Societies study. Osteoporos Sarcopenia 4:16–21. https://doi.org/10.1016/j.afos.2018.03.003
Pharmaceutical Services Programme (2021) Official Portal of Pharmaceutical Services Programme, Ministry of Health Malaysia. https://www.pharmacy.gov.my/v2/en. Accessed 21 Apr 2021
Fatoye F, Smith P, Gebrye T, Yeowell G (2019) Real-world persistence and adherence with oral bisphosphonates for osteoporosis: a systematic review. BMJ Open 9:e027049. https://doi.org/10.1136/bmjopen-2018-027049
Cornelissen D, Boonen A, Bours S et al (2020) Understanding patients’ preferences for osteoporosis treatment: the impact of patients’ characteristics on subgroups and latent classes. Osteoporos Int 31:85–96. https://doi.org/10.1007/s00198-019-05154-9
Zhu Y, Huang Z, Wang Y et al (2020) The efficacy and safety of denosumab in postmenopausal women with osteoporosis previously treated with bisphosphonates: a review. J Orthop Transl 22:7–13. https://doi.org/10.1016/j.jot.2019.08.004
Chau D, Becker DL, Coombes ME et al (2012) Cost-effectiveness of denosumab in the treatment of postmenopausal osteoporosis in Canada. J Med Econ 15:3–14. https://doi.org/10.3111/13696998.2012.737393
Darbà J, Kaskens L, Vilela FS, Lothgren M (2015) Cost-utility of denosumab for the treatment of postmenopausal osteoporosis in Spain. Clin Outcomes Res 7:105–117. https://doi.org/10.2147/CEOR.S78349
Hiligsmann M, Reginster JY (2011) Cost effectiveness of denosumab compared with oral bisphosphonates in the treatment of post-menopausal osteoporotic women in Belgium. Pharmacoeconomics 29:895–911. https://doi.org/10.2165/11539980-000000000-00000
Jönsson B, Ström O, Eisman JA et al (2011) Cost-effectiveness of Denosumab for the treatment of postmenopausal osteoporosis. Osteoporos Int 22:967–982. https://doi.org/10.1007/s00198-010-1424-x
Mori T, Crandall CJ, Ganz DA (2017) Cost-effectiveness of denosumab versus oral alendronate for elderly osteoporotic women in Japan. Osteoporos Int 28:1733–1744. https://doi.org/10.1007/s00198-017-3940-4
Parthan A, Kruse M, Yurgin N et al (2013) Cost effectiveness of denosumab versus oral bisphosphonates for postmenopausal osteoporosis in the US. Appl Health Econ Health Policy 11:485–497. https://doi.org/10.1007/s40258-013-0047-8
Yoshizawa T, Nishino T, Okubo I, Yamazaki M (2018) Cost-effectiveness analysis of drugs for osteoporosis treatment in elderly Japanese women at high risk of fragility fractures: comparison of denosumab and weekly alendronate. Arch Osteoporos 13:94. https://doi.org/10.1007/s11657-018-0509-6
Karnon J, Shafie AS, Orji N, Usman SK (2016) What are we paying for? A cost-effectiveness analysis of patented denosumab and generic alendronate for postmenopausal osteoporotic women in Australia. Cost Eff Resour Alloc 14:11. https://doi.org/10.1186/s12962-016-0060-5
Pongchaiyakul C, Nanagara R, Songpatanasilp T, Unnanuntana A (2020) Cost-effectiveness of denosumab for high-risk postmenopausal women with osteoporosis in Thailand. J Med Econ 23:776–785. https://doi.org/10.1080/13696998.2020.1730381
Zethraeus N, Borgström F, Ström O et al (2007) Cost-effectiveness of the treatment and prevention of osteoporosis - a review of the literature and a reference model. Osteoporos Int 18:9–23. https://doi.org/10.1007/s00198-006-0257-0
Ministry of Health Malaysia (2015) Clinical Guidance on Management of Osteoporosis, Second edi. Ministry of Health Malaysia, Malaysia
Ministry of Health Malaysia (2019) Pharmacoeconomic Guidelines for Malaysia, Second edi. Ministry of Health Malaysia, Malaysia
World Health Organization, Baltussen, Rob M. P. M, Adam, Taghreed, Tan-Torres Edejer, Tessa, Hutubessy, Raymond CW et al (2003) Making choices in health: WHO guide to cost-effectiveness analysis / edited by T. Tan-Torres Edejer ... [et al]. Geneva: World Health Organization
Hiligsmann M, Reginster JY, Tosteson ANA et al (2019) Recommendations for the conduct of economic evaluations in osteoporosis: outcomes of an experts’ consensus meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and. Osteoporos Int 30:45–57. https://doi.org/10.1007/s00198-018-4744-x
Husereau D, Drummond M, Augustovski F et al (2022) Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) Statement: updated reporting guidance for health economic evaluations. Pharmacoeconomics 2022https://doi.org/10.1007/s40273-021-01112-8
Medical Development Division (2021) Malaysian DRG Executive Information System. In: Minist. Heal. Malaysia. http://casemix-eis.moh.gov.my/. Accessed 1 Feb 2021
Klotzbuecher CM, Ross PD, Landsman PB et al (2000) Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 15:721–739. https://doi.org/10.1359/jbmr.2000.15.4.721
Fleurence RL, Hollenbeak CS (2007) Rates and probabilities in economic modelling: Transformation, translation and appropriate application. Pharmacoeconomics 25:3–6. https://doi.org/10.2165/00019053-200725010-00002
Department of Statistics Malaysia (2020) Vital Statistics, Malaysia, 2020. Malaysia
Chang CY, Tang CH, Chen KC et al (2016) The mortality and direct medical costs of osteoporotic fractures among postmenopausal women in Taiwan. Osteoporos Int 27:665–676. https://doi.org/10.1007/s00198-015-3238-3
Ha YC, Kim TY, Lee A et al (2016) Current trends and future projections of hip fracture in South Korea using nationwide claims data. Osteoporos Int 27:2603–2609. https://doi.org/10.1007/s00198-016-3576-9
Kwon GD, Jang S, Lee A et al (2016) Incidence and mortality after distal radius fractures in adults aged 50 years and older in Korea. J Korean Med Sci 31:630–634. https://doi.org/10.3346/jkms.2016.31.4.630
Kim TY, Jang S, Park CM et al (2016) Trends of incidence, mortality, and future projection of spinal fractures in Korea using nationwide claims data. J Korean Med Sci 31:801–805. https://doi.org/10.3346/jkms.2016.31.5.801
Li S, Sun T, Liu Z (2016) Excess mortality of 1 year in elderly hip fracture patients compared with the general population in Beijing, China. Arch Osteoporos 11:35. https://doi.org/10.1007/s11657-016-0289-9
Man LP, Angela Ho AWH, Wong SH (2016) Excess mortality for operated geriatric hip fracture in Hong Kong. Hong Kong Med J 22:6–10. https://doi.org/10.12809/hkmj154568
Kanis JA, Oden A, Johnell O et al (2003) The components of excess mortality after hip fracture. Bone 32:468–473. https://doi.org/10.1016/S8756-3282(03)00061-9
Kanis JA, Oden A, Johnell O et al (2004) Excess mortality after hospitalisation for vertebral fracture. Osteoporos Int 15:108–112. https://doi.org/10.1007/s00198-003-1516-y
Barrionuevo P, Kapoor E, Asi N et al (2019) Efficacy of pharmacological therapies for the prevention of fractures in postmenopausal women: a network meta-analysis. J Clin Endocrinol Metab 104:1623–1630. https://doi.org/10.1210/jc.2019-00192
Ström O, Borgström F, Kanis JA, Jönsson B (2009) Incorporating adherence into health economic modelling of osteoporosis. Osteoporos Int 20:23–34. https://doi.org/10.1007/s00198-008-0644-9
Reyes C, Tebe C, Martinez-Laguna D et al (2017) One and two-year persistence with different anti-osteoporosis medications: a retrospective cohort study. Osteoporos Int 28:2997–3004. https://doi.org/10.1007/s00198-017-4144-7
Hadji P, Kyvernitakis I, Kann PH et al (2016) GRAND-4: the German retrospective analysis of long-term persistence in women with osteoporosis treated with bisphosphonates or denosumab. Osteoporos Int 27:2967–2978. https://doi.org/10.1007/s00198-016-3623-6
Cheng LI, Durden E, Limone B et al (2015) Persistence and compliance with osteoporosis therapies among women in a commercially insured population in the United States. J Manag Care Pharm 21:824–833. https://doi.org/10.18553/jmcp.2015.21.9.824
Durden E, Pinto L, Lopez-Gonzalez L et al (2017) Two-year persistence and compliance with osteoporosis therapies among postmenopausal women in a commercially insured population in the United States. Arch Osteoporos 12:22. https://doi.org/10.1007/s11657-017-0316-5
Lakatos P, Takács I, Marton I et al (2016) A retrospective longitudinal database study of persistence and compliance with treatment of osteoporosis in Hungary. Calcif Tissue Int 98:215–225. https://doi.org/10.1007/s00223-015-0082-6
Morley J, Moayyeri A, Ali L et al (2020) Persistence and compliance with osteoporosis therapies among postmenopausal women in the UK Clinical Practice Research Datalink. Osteoporos Int 31:533–545. https://doi.org/10.1007/s00198-019-05228-8
Miyazaki T, Tokimura F, Tanaka S (2014) A review of denosumab for the treatment of osteoporosis. Patient Prefer Adherence 8:463–471. https://doi.org/10.2147/PPA.S46192
Bone HG, Hosking D, Devogelaer J-P et al (2004) Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 350:1189–1199. https://doi.org/10.1056/nejmoa030897
Black DM, Schwartz AV, Ensrud KE et al (2006) Effects of continuing or stopping alendronate after 5 years of treatment: The Fracture Intervention Trial long-term extension (FLEX): a randomized trial. J Am Med Assoc 296:2927–2938. https://doi.org/10.1001/jama.296.24.2927
McClung MR, Wagman RB, Miller PD et al (2017) Observations following discontinuation of long-term denosumab therapy. Osteoporos Int 28:1723–1732. https://doi.org/10.1007/s00198-017-3919-1
Kendler DL, McClung MR, Freemantle N et al (2011) Adherence, preference, and satisfaction of postmenopausal women taking denosumab or alendronate. Osteoporos Int 22:1725–1735. https://doi.org/10.1007/s00198-010-1378-z
Brown JP, Prince RL, Deal C et al (2009) Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res 24:153–161. https://doi.org/10.1359/jbmr.0809010
World Bank Group (2021) GDP deflator (base year varies by country) - Malaysia. https://data.worldbank.org/indicator/NY.GDP.DEFL.ZS?end=2019&locations=MY&start=2010. Accessed 15 Feb 2021
Central Bank of Malaysia (2021) Exchange Rates 2019. https://www.bnm.gov.my/exchange-rates. Accessed 1 Mar 2021
Federal Government Gazette (2014) Fees (Medical) (Cost of Services) Order 2014. Attorney General’s Chambers, Malaysia
Ara R, Brazier JE (2011) Using health state utility values from the general population to approximate baselines in decision analytic models when condition-specific data are not available. Value Heal 14:539–545. https://doi.org/10.1016/j.jval.2010.10.029
Si L, Winzenberg TM, De Graaff B, Palmer AJ (2014) A systematic review and meta-analysis of utility-based quality of life for osteoporosis-related conditions. Osteoporos Int 25:1987–1997. https://doi.org/10.1007/s00198-014-2636-2
Lim YW, Shafie AA, Chua GN, Ahmad Hassali MA (2017) Determination of cost-effectiveness threshold for health care interventions in Malaysia. Value Heal 20:1131–1138. https://doi.org/10.1016/j.jval.2017.04.002
Briggs A, Sculpher M, Claxton K (2006) Decision modelling for health economic evaluation. Oxford University Press
Funding
This study was funded by the Geran Galakan Penyelidik Muda (grant number GGPM-2019–054).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
YWC, MSMS, MMB, SCL, and NAMT declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Choo, Y.W., Mohd Tahir, N.A., Mohamed Said, M.S. et al. Cost-effectiveness of Denosumab for the Treatment of Postmenopausal Osteoporosis in Malaysia. Osteoporos Int 33, 1909–1923 (2022). https://doi.org/10.1007/s00198-022-06444-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-022-06444-5