Abstract
Summary
We investigated whether the drug denosumab modulates the inflammatory response after total hip arthroplasty in a randomized controlled trial. Significantly increased expression of RANKL was found in patients treated with denosumab. This could provide an explanation for the rebound effect with rapid loss of BMD seen after discontinuation of denosumab treatment.
Purpose
To evaluate whether denosumab, a human monoclonal antibody directed against receptor activator of nuclear factor kappa-B ligand (RANKL), modulates the inflammatory response after cementless total hip arthroplasty (THA) in patients with osteoarthritis of the hip.
Methods
Sixty-four patients operated with cementless THA were randomized to two doses of 60-mg denosumab or placebo 1–3 days and 6 months postoperatively. Serum samples were analyzed by a multiplex extension assay detecting 92 inflammation-related proteins. Bone turnover markers were assessed. Proteins were analyzed using linear mixed effect models. Validation of conspicuous findings was performed with ELISA.
Results
Two proteins were significantly affected by denosumab treatment: RANKL and tumor necrosis factor receptor super family member 9 (TNFRSF9). Serum levels of RANKL were more than twice as high in the denosumab than in the placebo group 3 months after surgery (ratio 2.10, p<0.001). Six and 12 months after surgery, the expression of RANKL was still elevated in the denosumab-treated group (ratios 1.50, p < 0.001; 1.47, p =0.002). The expression of TNFRSF9 was lower in the denosumab group at 3 months (ratio 0.68, p<0.001). In the denosumab group, concentrations of bone turnover markers were substantially reduced after 3 months, remained suppressed after 6 and 12 months, but increased above baseline at 24 months after surgery.
Conclusion
Two subcutaneous denosumab injections 6 months apart increase RANKL and depress TNFRSF9 after THA. This provides a possible explanation for the rebound effect on bone turnover markers as well as bone mineral density (BMD) upon withdrawal of denosumab. None of the other measured markers of inflammation was influenced by denosumab treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Implant loosening is the most common cause of total hip arthroplasty (THA) revision surgery worldwide, and it is well known that periprosthetic bone mineral density (BMD) decreases with age [1,2,3,4,5]. A number of drugs are known to prevent bone loss, enhance bone formation, increase BMD, and reduce the risk of osteoporosis-related fractures [6]. Several clinical studies have investigated whether such drugs can prevent periprosthetic bone loss after THA [7,8,9]. Bisphosphonates can reduce the risk of revision surgery after continuous usage [10] and are known to reduce periprosthetic bone loss immediately after surgery. However, the effect of pharmaceutical therapies on periprosthetic BMD seems to be transient [7,8,9].
Denosumab is a human monoclonal antibody directed against receptor activator of nuclear factor kappa-B ligand (RANKL). It inhibits osteoclast recruitment and activation and thereby rapidly suppresses biochemical markers of bone resorption and formation [11]. Subcutaneous injections of 60-mg denosumab every 6 months increase BMD and reduce the risk of osteoporotic fractures in patients with osteoporosis [12]. However, after treatment cessation, there is a rebound effect with increased biochemical markers for bone metabolism. Previous gains in BMD and protection from vertebral fractures are rapidly lost [13,14,15].
Proteomics is a technique for the analysis of protein biomarkers with high specificity and minimal sample volumes. OLINK Target 96 Inflammation® is a semiquantitative method to analyze biomarkers possibly related to inflammation. Among the proteins included in OLINK Target 96 inflammation® are TRANCE, also known as RANKL [11], and TNF receptor super family member 9 (TNFRSF9) also known as CD137 and 4-1BB [16,17,18].
We have performed a randomized, double-blind placebo-controlled clinical trial (RCT) in middle-aged patients (35 to 65 years) with unilateral osteoarthritis of the hip (OAH) needing a THA. The patients were given two doses of denosumab, a human monoclonal antibody directed against RANKL, or placebo. The primary outcome variable, femoral periprosthetic BMD within 2 years after surgery, has been reported previously [19]. In summary, denosumab potently inhibited periprosthetic bone loss. After 12 months, BMD in the denosumab group was 32% (95% confidence interval [CI] 22–44) higher in Gruen zone 7 and 11% (95% CI 8–15) in zones 1–7. After 24 months, the difference in BMD between groups had decreased to 15% (95% CI 4–27) in zone 7 and 4% in (95% CI 0–8) in zones 1–7. In that study, bone turnover markers (C-terminal telopeptide of type 1 collagen (CTX) and serum procollagen type 1N propeptide (P1NP)) were significantly lower in the denosumab group 3 months after surgery. They remained depressed after 6 and 12 months until a rebound effect in the denosumab group at 24 months, 12 months after denosumab was discontinued.
The present study aims to evaluate whether denosumab modulates the inflammatory response after a cementless THA in patients with OAH.
Materials and methods
Trial design
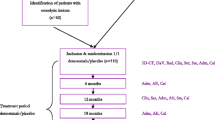
The primary and selected secondary outcomes of the trial have been described in detail previously [19]. A prospective, randomized, double-blind, placebo-controlled phase two clinical trial was performed in patients with unilateral OAH that underwent cementless THA to investigate whether a RANKL-inhibitor could inhibit periprosthetic BMD loss that regularly occurs after such interventions [1].
Sixty-four patients were randomized to either two subcutaneous doses of denosumab (n=32) or placebo (n=32) given 1–3 days and 6 months after surgery. Patients were followed for 24 months. Morning fasting blood samples were collected 7–14 days before surgery, 1–3 days and 3, 6, 12, and 24 months after surgery, resulting in six sequential samples from each patient. Given the reported outcome of the trial, we decided to investigate potential differences in the inflammatory response between patients treated with denosumab and placebo.
The trial was registered at ClinicalTrials.gov 2011-001481-18, NCT01630941 and approved by the Regional Ethics Committee in Uppsala, Sweden (2011/297/3).
Analysis of inflammatory response–related proteins
Serum samples were analyzed by a multiplex extension assay detecting 92 inflammation-related proteins (Olink Proteomics, Uppsala, Sweden) and bone turnover markers (P1NP and CTX).
Participants
All patients (n=461) aged 35 to 65 years, referred to the Department of Orthopaedics, Uppsala University Hospital, were assessed for eligibility between 7 August 2012 and 21 January 2015. Inclusion criteria were radiologically verified OAH defined as Kellgren-Lawrence (KL) grades 3–4 of the affected hip. Exclusion criteria were contralateral OAH above KL grade 1 or previous THA, bodyweight >110 kg or body mass index (BMI) >35 kg/m2, treatment with bone-modulating drugs or corticosteroids, malignancy, American Society of Anesthesiologists (ASA) class >3, substance abuse, pregnancy, previous exposure to large doses of irradiation, or otherwise unsuitable as judged by the investigators; for details, see Nyström et al. [19].
Randomization and blinding
In all, 64 patients were found eligible and started on a daily treatment of calcium (500mg) and vitamin D3 (800IE) 7–14 days before the procedure and up to 1 year. The sealed-envelope technique was used to randomize patients to receive an injection of either 60 mg of denosumab or a placebo (0.9% saline). A detailed description of the procedure appears in Nyström et al. [19].
Peri- and postoperative procedures and implants
Cementless THA was performed by one of two surgeons using a Continuum cup with a highly crossed-linked polyethylene-elevated liner, a collum femoris–preserving stem, and a 28-mm CoCr head. The subcutaneous injections of denosumab or placebo were given after baseline dual-energy X-ray absorptiometry (DXA). Fasting morning blood samples were drawn 1–3 days postoperatively, prior to the first administration of denosumab. The second injections were given 6 months later.
Biochemical markers of bone metabolism
One to 2 ml of morning fasting blood samples were collected, serum prepared, and stored at −70°C. Carboxy-terminal telopeptide of type 1 collagen (CTX, β-CrossLaps, Cobas, Roche, Rotkreuz, Switzerland) and procollagen type 1 amino-terminal propeptide (P1NP, Cobas, Roche, Rotkreuz, Switzerland) were quantified as a reference for bone metabolism/formation [20]. Coefficient of variation (CV) analysis was performed (3% for P1NP and 6% for CTX, lab certified in accordance with ISO 15189:201).
Proximity extension assay
Serum samples from preoperatively 3, 6, and 12 months were analyzed using multiplexed proximity extension assay (PEA) immunoassay using Proseek Multiplex inflammation panel (Olink Proteomics, Uppsala, Sweden). This procedure allows for the simultaneous analysis of 92 inflammation-related proteins across 96 samples [21, 22, suppl]. One μl of serum samples or controls was prepared in 96 well plates mixed with 3 μl of a solution containing 92 DNA-oligonucleotide-conjugated antibodies and incubated overnight. Next, a solution containing PEA enzyme and the reagents for polymerase chain reaction (PCR) were added to the mixture, incubated for 5 min at room temperature, and transferred to a microfluidic real-time quantitative PCR BioMark HD System (Fluidigm, San Francisco, USA) for amplification according to instructions from ProSeek Multiplex. The data were then corrected and expressed on a log2 scale by normalizing quantification cycle values against controls and a correction factor. Limits of detection were defined as three standard deviations above the background.
Enzyme-linked immunosorbent assay
The concentration of RANKL was measured using a commercial sandwich enzyme-linked immunosorbent assay (ELISA, DY626, R&D Systems, Minneapolis, MN, USA) to validate the results obtained by PEA on RANKL. The total CV for the assay was approximately 6%, and testing was performed blinded, without information on clinical data. The ELISA evaluation of RANKL also included visits two and six (i.e., all visits).
Bone mineral density
All scans were performed on a Prodigy Advance system. Preoperatively, the lumbar spine and proximal femora were scanned in an anteroposterior (AP) view. Postoperatively, measurements of periprosthetic BMD, lumbar spine, and the unaffected hip were investigated during the 24-month follow-up. For details, see Nyström et al. [19].
Conventional radiography
AP pelvis and AP/lateral hip views were performed preoperatively, with bilateral classification of OAH according to the KL system [23].
Statistical analysis
Descriptive statistics were used to compare baseline characteristics of the trial participants. Of the 92 proteins, 17 had more than 20% values below the limit of detection. These 17 proteins were excluded, leaving 74 for further analysis (in four proteins the lower limit of detection replaced values below this limit).
The proteins were then studied one at a time in linear mixed effect models. The protein level was set as the dependent variable. The independent variables with fixed effects were age, sex, BMI, plate, visit, treatment, and the interaction between visit and treatment. Patient identity was considered a random effect. Likelihood ratio tests estimated the effect of visit, treatment, and the interaction of visit and treatment on the protein level after adjusting for age, sex, BMI, and plate. The test results in a p value per protein, the global p value. Because the 74 tests cannot be considered independent (the proteins were selected because they are all involved in inflammation), we assume and adjust for 50 independent tests instead of 74. The Bonferroni method was applied to control the family-wise error rate to 5% to adjust for multiple testing. This will correspond to a significance threshold of 0.001 (0.05/50). All statistically significant proteins were further analyzed in post hoc analyses. We applied the Tukey test for pairwise post hoc testing, and p<0.05 was chosen as the error level. All statistical analyses were performed using R Statistical Software (version 3.6.3; R Foundation for Statistical Computing, Vienna, Austria).
Results
Change in inflammatory markers
Two proteins were significantly affected by denosumab treatment: RANKL (TRANCE) and TNFRSF9 (CD137/4-1BB). In the denosumab-treated patients, the expression of RANKL after 3 months was more than twice as high compared to the placebo group (ratio denosumab/placebo=2.10, p<0.001). Six months after surgery and immediately before the second dose of denosumab, the expression of RANKL remained 50% higher in the group treated with denosumab (ratio denosumab/placebo=1.50, p <0.001). After 12 months postoperatively, this difference persisted to about the same extent (ratio denosumab/placebo=1.47, p=0.002) (Fig. 1).
Protein levels at visit 0 days, 3, 6, and 12 months for RANKL and TNFRFS9. The protein level is expressed in NPX (normalized protein expression) a relative value on log2 scale. Boxplots summarize the data, where the box ranges from first to third quartile, with the median indicated in the middle. The whiskers range from minimum to maximum value within 1.5 IQR (inter-quartile range) from the border of the box. Any values further out, so called outliers, are represented by dots
The level of TNFRSF9 decreased statistically significant in patients treated with denosumab 3 months after surgery but returned to baseline level at 6 months (Fig. 1). In contrast, the placebo group TNFRSF9 increased at 3 months before gradually returning to the baseline level. In pairwise comparisons between denosumab and placebo a significant reduction of TNFRSF9 was seen in the group treated with denosumab 3 months after surgery compared to placebo (ratio denosumab/placebo 0.68, p<0.001). The difference, although smaller, remained significant after 6 (ratio denosumab/placebo 0.83, p=0.004) and 12 months postoperatively (ratio denosumab/placebo 0.86, p=0.026).
Validation using ELISA
Since we found an increased expression of RANKL in the denosumab group, we validate this finding derived from the PEA assay by use of an ELISA for the RANKL protein. A similar increase in protein content as in RANKL expression was found in patients treated with denosumab compared to placebo, but the only time point with a statistically significant elevation of RANKL was 3 months after surgery (Fig. 2). Here, RANKL concentrations were 2.68 times higher in the group treated with denosumab (p=0.038).
RANKL concentration on log2 scale at the different visits as measured by ELISA. Boxplots summarize the data, where the box ranges from first to third quartile, with the median indicated in the middle. The whiskers range from minimum to maximum value within 1.5 IQR (inter-quartile range) from the border of the box. Any values further out are, so called outliers, are represented by dots
Discussion
Principal findings
As a secondary endpoint analysis of a previous RCT in which patients were randomized to denosumab or placebo to study effects on periprosthetic BMD [19], we now analyzed serum levels of markers of inflammation in this cohort. We found that RANKL was strongly upregulated throughout the observation period in patients receiving denosumab treatment, with the largest effect size measured 3 months after the start of treatment. Conversely, TNFRSF9 was downregulated in patients in the denosumab group 3 months after treatment initiation. However, levels of TNFRSF9 returned to near baseline already 6 months after initiation of treatment. For the other investigated markers, no statistically significant differences were found between the denosumab and placebo groups.
An inflammatory component in OAH has previously been suggested [24]. When OAH patients were treated with a THA, there is a marked improvement in patient-reported outcome measures [19], but this was not reflected or accompanied by significant changes in the inflammatory markers selected in the Olink Proseek Multiplex inflammation panel.
Strengths and weaknesses of the study
A major strength of the study is that it is derived on a rigorously conducted RCT. The patients’ important baseline characteristics in this trial are thus similar between treatment arms, which is especially important for pre-operative BMD [19]. The study population is also well-defined, representing patients with advanced unilateral OAH without prior bone metabolic disorders and previous or ongoing medication that could influence bone metabolism or inflammation, such as glucocorticoids or other antiresorptive treatments. Sixty-three of 64 patients completed the 24-month follow-up, and 61 patients had serum samples collected at the 24-month follow-up.
A major weakness of this study is that our investigation of inflammatory markers by PEA and ELISA is a post hoc analysis. Thus, the current analysis is based on the sample size derived from a power calculation designed to investigate periprosthetic BMD, not the numbers needed to investigate an a priori question related to changes in inflammatory markers. For most of the investigated molecules, we found no statistically significant differences between treatment arms, raising questions about the presence of type II errors. Another weakness of our study is that we only validated PEA-derived findings on changes in RANKL by measuring actual serum concentrations by ELISA. Only RANKL was substantially influenced by denosumab treatment, whereas the TNF-receptor TNFRSF9 was down-regulated only after 3 months but not later, and concentrations of other investigated molecules did not differ between treatment arms.
The unexpected finding of elevated levels of RANKL raises questions whether the RANKL-denosumab complex could interfere with the PEA measurement. There is always a chance that an antibody binds unspecific to another protein. However, PEA technology builds on the fact that a pair of antibodies binds to the protein and a unique DNA sequence can be measured, thus cross-events should not be detected, in contrast to traditional antibody-based technologies [22]. Also, the elimination half-life of 60-mg denosumab ranges between 15 and 32 days, thus being shorter than the first measured time point after 3 months [25, 26]. When an antibody is bound to an antigen an immune complex is formed. Depending on the size of the complex, it is cleared more rapidly than a free antibody. As denosumab is foreign to the immune system, there will be a response with production of anti-idiotypic antibodies that will further shorten the half-life of denosumab. If denosumab blocks the binding of the assay antibody to the immunogen, the result should be lower at 3 months. Taken together, it is expected that at least 90% of the administered denosumab dose has been cleared after 3 months.
Unfortunately, the PEA was only performed on four different occasions, preoperatively, 3, 6, and 12 months after surgery. The increased levels of RANKL were unexpected, and seen in retrospect, it would have been valuable to obtain PEA data also at the 24-months follow-up.
Although RANKL was found to be higher 3 months after surgery, both by PEA analysis and ELISA measurement, the differences were not statistically significant at 6 or 12 months. However, the log-fold change in RANKL concentrations measured by ELISA shows a similar temporal trend (supplementary material). RANKL can be expressed in at least three different isoforms, thus there is a great variability in what is termed RANKL. It is not certain that kits from different manufacturers measure the exact same form of RANKL. Further studies are warranted to more precisely define the exact specificity of these assays.
Some important strengths but also limitations are revealed when comparing our study to others. Again, one strength lies in the RCT design of our study. Other studies investigating changes in inflammatory markers or RANKL concentrations during or after denosumab treatment involve retrospective analyses, with or without control groups [27, 28]. Another strength is our use of the multiplexed PEA that enabled us to simultaneously analyze a large number of molecules associated with inflammation and pain, rather than restricting our analysis to a few candidate molecules, but only in relative and not absolute measures [29]. Although our sample size was limited, our cohort is still comparable to those studied by other authors interested in the rebound phenomenon after denosumab treatment [29].
In an impressive prospective longitudinal cohort, Cassuto et al. measured inflammatory markers after THA. They presented a primary peak in proinflammatory cytokine expression that we were unable to confirm. In addition, they reported a peak of RANKL concentrations at 5 years, but before that, levels were lower than baseline. Those early results are in line with findings in our placebo group [30,31,32].
Fassio et al. [28] recently published a prospective cohort study of 15 patients with discontinuation of long-term denosumab treatment and a follow-up of 12 months. These authors evaluated RANKL levels in relation to the canonical WNT pathway with the presentation of BMD, bone turnover markers, and WNT inhibitors. In contrast to our findings, Fassio et al. saw an increase of RANKL after discontinuation of denosumab treatment. Unfortunately, in that study, the levels of all serum markers, including RANKL, were missing not only at baseline but also during the denosumab treatment. Moreover, RANKL concentrations in serum increased together with those of P1NP and CTX, whereas concentrations of Dickkopf (Dkk)-1 and sclerostin decreased. Taken together, these changes suggest that the observed rebound phenomenon can be ascribed to hyperstimulation of osteoclasts after disinhibition. However, there are some limitations when comparing this material to our findings. The indication for denosumab treatment in the cited study was postmenopausal osteoporosis, there was a lack of control group, and the sample size was small. These contradicting findings raise the need for further studies in the field.
McDonald et al. recently published evidence that, instead of undergoing apoptosis, osteoclasts can fission into osteomorphs, recycling to osteoclasts under RANKL stimulation [33]. Inhibiting RANKL blocked this recycling process and resulted in the accumulation of osteomorphs. Our findings indicate that a compensatory upregulation of RANKL occurred already directly after the initiation of denosumab treatment and was sustained during the entire treatment period. Thus, it is tempting to speculate that if withdrawal of denosumab occurs when molecules that stimulate osteoclastogenesis are abundant, there will be rapid recruitment of osteoclasts and recycling of osteomorphs — the net effect being substantially increased bone resorption.
The TNFRSF9, believed to enhance RANKL-induced osteoclastogenesis, is expressed on osteoclast precursors [16, 17, 34, 35]. The downregulation of this receptor found in our study may be a direct effect of denosumab treatment or part of a complex feedback loop triggered by the blocking of RANK receptors. This view supports our findings indicating bidirectional signaling between RANKL and TNFRSF9 for maintaining cellular homeostasis and osteoclastogenesis.
Conclusion
We found that RANKL was upregulated during the first 12 months after denosumab treatment, with the largest effect size measured 3 months after the initiation of treatment. Contrarily, TNFRFS9, was downregulated in patients in the intervention group. None of the other measured markers of inflammation were influenced by denosumab treatment. Our findings indicate that this compensatory upregulation of RANKL can be coupled via reverse signaling to TNFRFS9. Combined with novel insights into the regulation of osteomorphs, these changes may partly explain the immediate rebound effect seen on BMD after discontinuance of denosumab treatment.
References
Knutsen AR, Lau N, Longjohn DB, Ebramzadeh E, Sangiorgio SN (2017) Periprosthetic femoral bone loss in total hip arthroplasty: systematic analysis of the effect of stem design. HIP Int 27:26–34. https://doi.org/10.5301/hipint.5000413
Goodman SB, Gallo J (2019) Periprosthetic osteolysis: mechanisms, prevention and treatment. J Clin Med 8:2091. https://doi.org/10.3390/jcm8122091
Gallo J, Kaminek P, Ticha V, Rihakova P, Ditmar R (2002) Particle disease. A comprehensive theory of periprosthetic osteolysis: a review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 146:21–28
Pajarinen J, Jamsen E, Konttinen YT, Goodman SB (2014) Innate immune reactions in septic and aseptic osteolysis around hip implants. J Long-Term Eff Med Implants 24:283–296
Sköldenberg OG, Bodén HSG, Salemyr MOF, Ahl TE, Adolphson PY (2006) Periprosthetic proximal bone loss after uncemented hip arthroplasty is related to stem size: DXA measurements in 138 patients followed for 2–7 years. Acta Orthop 77:386–392. https://doi.org/10.1080/17453670610046307
Black DM, Rosen CJ (2016) Clinical practice. Postmenopausal osteoporosis. N Engl J Med 374:254–262. https://doi.org/10.1056/NEJMcp1513724
Sköldenberg OG, Salemyr MO, Bodén HS, Ahl TE, Adolphson PY (2011) The effect of weekly risedronate on periprosthetic bone resorption following total hip arthroplasty: a randomized, double-blind, placebo-controlled trial. J Bone Joint Surg Am 93:1857–1864. https://doi.org/10.2106/JBJS.J.01646
Zhou W, Liu Y, Guo X, Yang H, Xu Y, Geng D (2019) Effects of zoledronic acid on bone mineral density around prostheses and bone metabolism markers after primary total hip arthroplasty in females with postmenopausal osteoporosis. Osteoporos Int 30:1581–1589. https://doi.org/10.1007/s00198-019-05005-7
Kobayashi N, Inaba Y, Uchiyama M, Ike H, Kubota S, Saito T (2016) Teriparatide versus alendronate for the preservation of bone mineral density after total hip arthroplasty – a randomized controlled trial. J Arthroplast 31:333–338. https://doi.org/10.1016/j.arth.2015.07.017
Khatod M, Inacio MCS, Dell RM, Bini SA, Paxton EW, Namba RS (2015) Association of bisphosphonate use and risk of revision after THA: outcomes from a US total joint replacement registry. Clin Orthop 473:3412–3420. https://doi.org/10.1007/s11999-015-4263-4
Delmas PD (2008) Clinical potential of RANKL inhibition for the management of postmenopausal osteoporosis and other metabolic bone diseases. J Clin Densitom Off J Int Soc Clin Densitom 11:325–338. https://doi.org/10.1016/j.jocd.2008.02.002
Cummings SR, Martin JS, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361:756–765. https://doi.org/10.1056/NEJMoa0809493
Anastasilakis AD, Polyzos SA, Makras P, Aubry-Rozier B, Kaouri S, Lamy O (2017) Clinical features of 24 patients with rebound-associated vertebral fractures after denosumab discontinuation: systematic review and additional cases. J Bone Miner Res Off J Am Soc Bone Miner Res 32:1291–1296. https://doi.org/10.1002/jbmr.3110
Söderström LÅ, Tarnawski L, Olofsson PS (2018) CD137: a checkpoint regulator involved in atherosclerosis. Atherosclerosis 272:66–72. https://doi.org/10.1016/j.atherosclerosis.2018.03.007
Tsourdi E, Zillikens MC, Meier C, Body J-J, Gonzalez Rodriguez E, Anastasilakis AD, Abrahamsen B, McCloskey E, Hofbauer LC, Guañabens N, Obermayer-Pietsch B, Ralston SH, Eastell R, Pepe J, Palermo A, Langdahl B (2020) Fracture risk and management of discontinuation of denosumab therapy: a systematic review and position statement by ECTS. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgaa756
Yang J, Park OJ, Lee YJ, Jung H-M, Woo KM, Choi Y (2008) The 4-1BB ligand and 4-1BB expressed on osteoclast precursors enhance RANKL-induced osteoclastogenesis via bi-directional signaling. Eur J Immunol 38:1598–1609. https://doi.org/10.1002/eji.200737650
Senthilkumar R, Lee H-W (2009) CD137L- and RANKL-mediated reverse signals inhibit osteoclastogenesis and T lymphocyte proliferation. Immunobiology 214:153–161. https://doi.org/10.1016/j.imbio.2008.05.001
Tang Q, Jiang D, Harfuddin Z, Cheng K, Moh MC, Schwarz H (2014) Regulation of myelopoiesis by CD137L signaling. Int Rev Immunol 33:454–469. https://doi.org/10.3109/08830185.2014.921163
Nyström A, Kiritopoulos D, Ullmark G, Sörensen J, Petrén-Mallmin M, Milbrink J, Hailer NP, Mallmin H (2020) Denosumab prevents early periprosthetic bone loss after uncemented total hip arthroplasty: results from a randomized placebo-controlled clinical trial. J Bone Miner Res 35:239–247. https://doi.org/10.1002/jbmr.3883
for the National Bone Health Alliance Bone Turnover Marker Project, Szulc P, Naylor K, Hoyle NR, Eastell R, Leary ET (2017) Use of CTX-I and PINP as bone turnover markers: National Bone Health Alliance recommendations to standardize sample handling and patient preparation to reduce pre-analytical variability. Osteoporos Int 28:2541–2556. https://doi.org/10.1007/s00198-017-4082-4
Lundberg M, Eriksson A, Tran B, Assarsson E, Fredriksson S (2011) Homogeneous antibody-based proximity extension assays provide sensitive and specific detection of low-abundant proteins in human blood. Nucleic Acids Res 39:e102–e102. https://doi.org/10.1093/nar/gkr424
Assarsson E, Lundberg M, Holmquist G, Björkesten J, Bucht Thorsen S, Ekman D, Eriksson A, Rennel Dickens E, Ohlsson S, Edfeldt G, Andersson A-C, Lindstedt P, Stenvang J, Gullberg M, Fredriksson S (2014) Homogenous 96-Plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One 9:e95192. https://doi.org/10.1371/journal.pone.0095192
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16:494–502. https://doi.org/10.1136/ard.16.4.494
Katz JN, Arant KR, Loeser RF (2021) Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA 325:568–578. https://doi.org/10.1001/jama.2020.22171
Narayanan P (2013) Denosumab: a comprehensive review. South Asian J Cancer 2:272–277. https://doi.org/10.4103/2278-330X.119895
Chen Q, Hu C, Liu Y, Song R, Zhu W, Zhao H, Nino A, Zhang F, Liu Y (2018) Pharmacokinetics, pharmacodynamics, safety, and tolerability of single-dose denosumab in healthy Chinese volunteers: a randomized, single-blind, placebo-controlled study. PLoS One 13:e0197984. https://doi.org/10.1371/journal.pone.0197984
Anastasilakis AD, Yavropoulou MP, Makras P, Sakellariou GT, Papadopoulou F, Gerou S, Papapoulos SE (2017) Increased osteoclastogenesis in patients with vertebral fractures following discontinuation of denosumab treatment. Eur J Endocrinol 176:677–683. https://doi.org/10.1530/EJE-16-1027
Fassio A, Adami G, Benini C, Vantaggiato E, Saag KG, Giollo A, Lippolis I, Viapiana O, Idolazzi L, Orsolini G, Rossini M, Gatti D (2019) Changes in Dkk-1, sclerostin, and RANKL serum levels following discontinuation of long-term denosumab treatment in postmenopausal women. Bone 123:191–195. https://doi.org/10.1016/j.bone.2019.03.019
Cipriani C, Piemonte S, Colangelo L, De Martino V, Diacinti D, Ferrone F, Piazzolla V, Fassino V, Nieddu L, Minisola S, Pepe J (2021) Inhibition of the RANKL with denosumab has no effect on circulating markers of atherosclerosis in women with postmenopausal osteoporosis: a pilot study. Endocrine 71:199–207. https://doi.org/10.1007/s12020-020-02483-2
Cassuto J, Folestad A, Göthlin J, Malchau H, Kärrholm J (2018) The key role of proinflammatory cytokines, matrix proteins, RANKL/OPG and Wnt/β-catenin in bone healing of hip arthroplasty patients. Bone 107:66–77. https://doi.org/10.1016/j.bone.2017.11.004
Anastasilakis AD, Makras P, Yavropoulou MP, Tabacco G, Naciu AM, Palermo A (2021) Denosumab discontinuation and the rebound phenomenon: a narrative review. J Clin Med 10:152. https://doi.org/10.3390/jcm10010152
Tsourdi E, Langdahl B, Cohen-Solal M, Aubry-Rozier B, Eriksen EF, Guañabens N, Obermayer-Pietsch B, Ralston SH, Eastell R, Zillikens MC (2017) Discontinuation of Denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone 105:11–17. https://doi.org/10.1016/j.bone.2017.08.003
McDonald MM, Khoo WH, Ng PY, Xiao Y, Zamerli J, Thatcher P, Kyaw W, Pathmanandavel K, Grootveld AK, Moran I, Butt D, Nguyen A, Corr A, Warren S, Biro M, Butterfield NC, Guilfoyle SE, Komla-Ebri D, Dack MRG et al (2021) Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell 184:1330–1347.e13. https://doi.org/10.1016/j.cell.2021.02.002
Saito K, Ohara N, Hotokezaka H, Fukumoto S, Yuasa K, Naito M, Fujiwara T, Nakayama K (2004) Infection-induced up-regulation of the costimulatory molecule 4-1BB in osteoblastic cells and its inhibitory effect on M-CSF/RANKL-induced in vitro osteoclastogenesis. J Biol Chem 279:13555–13563. https://doi.org/10.1074/jbc.M303791200
Jiang P, Gao W, Ma T, Wang R, Piao Y, Dong X, Wang P, Zhang X, Liu Y, Su W, Xiang R, Zhang J, Li N (2019) CD137 promotes bone metastasis of breast cancer by enhancing the migration and osteoclast differentiation of monocytes/macrophages. Theranostics 9:2950–2966. https://doi.org/10.7150/thno.29617
Acknowledgements
We want to thank Catharina Strömstedt for her excellent work as a research nurse and Demostenis Kiritopoulos and Andreas Nyström for their superb performance as main investigators in the original DATA study. We would like to thank the Berzelii project, Region Uppsala (ALF-grant and R&D funds), Uppsala University (R & R&D funds) and Skobranschens Utvecklingsfond, who, together with all the participants, made this study possible.
Funding
Open access funding provided by Uppsala University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Nils P Hailer has received institutional grants and lecturer’s fees from Waldemar Link GmbH and Heraeus, and institutional grants from Zimmer Biomet. None of the authors have had any other relationships or activities that could appear to have influenced the submitted work. Caroline Sköld, Kim Kultima, Eva Freyhult, Anders Larsson, Torsten Gordh, Nils P Hailer, and Hans Mallmin declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.

198_2022_6423_Fig3_ESM.png
Supplementary file1 (PNG 62 kb) Suppl fig 1 Log fold change in protein levels at visit 3, 6 and 12 months compared to visit 0 for RANKL and TNFRFS9. The protein level is expressed in NPX (normalized protein expression) a relative value on log2 scale. Boxplots summarize the data, where the box ranges from first to third quartile, with the median indicated in the middle. The whiskers range from minimum to maximum value within 1.5 IQR (inter-quartile range) from the border of the box. Any values further out, so called outliers, are represented by dots.

198_2022_6423_Fig4_ESM.png
Supplementary file2 (PNG 50 kb) Suppl Fig. 2 Log fold change of RANKL concentration on log2 scale at the different visits as measured by ELISA. Boxplots summarize the data, where the box ranges from first to third quartile, with the median indicated in the middle. The whiskers range from minimum to maximum value within 1.5 IQR (inter-quartile range) from the border of the box. Any values further out are, so called outliers, are represented by dots.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sköld, C., Kultima, K., Freyhult, E. et al. Effects of denosumab treatment on the expression of receptor activator of nuclear kappa-B ligand (RANKL) and TNF-receptor TNFRSF9 after total hip arthroplasty—results from a randomized placebo-controlled clinical trial. Osteoporos Int 33, 1–8 (2022). https://doi.org/10.1007/s00198-022-06423-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-022-06423-w