Abstract
Summary
This study demonstrates a substantial and persistent anti-osteoporosis treatment gap in men and women ≥50 years old who sustained major osteoporotic fracture(s) between 2005 and 2014 in Denmark. This was not substantially reduced by including hospital-administered anti-osteoporosis treatments. Strengthened post-fracture organization of care and secondary fracture prevention is highly needed.
Introduction
The purpose of this study was to evaluate the Danish anti-osteoporosis treatment gap from 2005 to 2014 in patients sustaining a major osteoporotic fracture (MOF), and to assess the impact of including hospital-administered anti-osteoporosis medications (AOM) on the treatment gap among these patients.
Methods
In this retrospective, registry-based study, we included men and women aged 50 years or older and living in Denmark, who sustained at least one MOF between 2005 and 2014. We applied a repeated cross-sectional design to generate cohorts of patients sustaining a first MOF, hip, vertebral, humerus, or forearm fracture, respectively, within each calendar year. We evaluated the treatment gap as the proportion of patients within each cohort not receiving treatment with AOM within 1 year of the fracture. Hospital-administered AOM was identified by SKS code.
Results
The treatment gap among MOF patients decreased from 85% in 2005 to 79% in 2014. The gap was smaller among hip and vertebral fracture patients as compared to humerus and forearm fracture patients, and it was smaller in women than in men. The use of hospital-administered AOM was relatively uncommon, with a maximum of 0.9% of MOF patients initiating hospital-administered AOM (in 2012). We observed substantial variations in this proportion between fracture types and gender. Hospital-administered AOM was most commonly used among vertebral fracture patients.
Conclusion
A significant treatment gap among patients sustaining a major osteoporotic fracture was present throughout our analysis, and including hospital-administered AOM did not significantly improve the treatment gap assessment. Improved secondary fracture prevention is urgently needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a highly prevalent disease, particularly among the older population, and is characterized by low bone mineral density (BMD), impaired bone microarchitecture, and an increased risk of fractures [1, 2]. In Denmark, the incidence rates of fractures in men and women aged 50 years or older have been found to be 170 and 320 per 10,000 patient-years for all fractures, and 78 and 200 per 10,000 patient-years for major osteoporotic fractures (MOF, a composite of hip, vertebral, humerus, and forearm fractures), respectively [3].
Patients with a previous osteoporotic fracture face an increased risk of subsequent fractures [4], most excessively elevated in the first years after the index fracture [5, 6]. Non-surprisingly, it has been reported that up to 45% of all hip fracture patients have had a prior fracture, and a study in Danish hip fracture patients demonstrated that 28% had sustained a MOF within the past 10 years [7,8,9]. The potential of anti-osteoporosis medications (AOM) to disrupt this fracture cascade by reducing subsequent fracture risk has been demonstrated [10]. Hence, Danish guidelines—generally in accordance with international guidelines—recommend assessment for osteoporosis in patients with any fragility fracture, while recognizing vertebral and proximal hip fractures as highly suggestive of osteoporosis and thus mandating AOM therapy unless disqualifying circumstances prevail [11,12,13,14,15,16].
Despite these recommendations, the majority of fragility fracture patients are not initiated on AOM, as has been demonstrated consistently across settings and healthcare systems [17,18,19,20,21,22,23,24,25,26]. This post-fracture treatment gap seems to be more pronounced in men [24,25,26]. We have recently published a study of the changing treatment gap from 2005 to 2015 across the United Kingdom, Catalonia, and Denmark, which confirmed the continuing existence of this treatment gap—with a persistent treatment gap of approximately 88–90% in Denmark—among patients with any first incident osteoporotic fracture [27]. We have only identified one treatment gap study attempting to identify the use of hospital-administered AOM [28], and only few studies reporting the use of AOM which in Denmark would be administered at a hospital—zoledronic acid and potentially denosumab—in their treatment gap assessment [19, 29]. Not including hospital-administered AOM could inflate treatment gap estimates but capturing such infusions and injections at the individual patient level is surprisingly challenging in most healthcare systems. It is therefore unclear if substantial reductions in the treatment gap may in fact already have been achieved through increased use of hospital-administered AOM.
In this paper we therefore aim to evaluate the Danish anti-osteoporosis treatment gap from 2005 to 2014 in annualized cohorts of patients sustaining a MOF, and to assess the impact of including hospital-administered AOM on the treatment gap among these patients.
Methods
This is a post hoc analysis of Danish data from the Multinational Observational Database Study on Imminent Osteoporotic Fracture Risk (the IFRISK study), which is a register-based study evaluating short-term fracture risk in patients at high risk of osteoporotic fractures [30]. In Denmark, the study was approved by the Danish Medicines Agency, the Danish Data Protection Agency, and Statistics Denmark (ref. number 706638). For this type of studies, ethical committee approval is not required.
In this retrospective analysis, we applied a repeated cross-sectional design to evaluate the post-fracture anti-osteoporosis treatment gap in consecutive annualized cohorts of patients sustaining an incident MOF from 2005 to 2014. Furthermore, we assessed—as part of this analysis—the impact of hospital-administered AOM on the treatment gap. The treatment gap was defined as the proportion of fracture patients not treated with AOM within 1 year of the index fracture. We performed the analyses separately for MOF and for each of the components of the composite MOF outcome, respectively.
To better understand the AOM treatment initiation and treatment gap, we also evaluated if any baseline covariates where associated with an increased likelihood of initiating AOM treatment. In addition, we also evaluated treatment persistence, defined as the proportion of patients who remained on AOM treatment one year after the first AOM treatment administration following the index fracture.
Data sources
We utilized data from the Danish Health Registries. This included the National Patient Register, which contains information on all admissions to public Danish hospitals since 1977 and out-patient visits since 1995, including diagnosis codes and treatments; the National Cause of Death Register, giving date and cause of death from 1970 and onwards; and the National Prescription Database, which contains information on all filled prescriptions from 1995. These registries cover the entire Danish population until death or migration.
Study population
Eligible for inclusion were men and women with incident hip, vertebral, humerus, and/or forearm fractures between 2005 and 2014, aged 50 years or older at the time of the fracture. Any first incident hip, vertebral, humerus, and forearm fracture in a given calendar year (= index fracture) qualified for inclusion in the respective annual cohort for that fracture type, while only the first of these fractures within a given calendar year would qualify for inclusion in the respective annual MOF cohort. If a fracture had been coded for the same anatomical location within the past 6 months, the new fracture code would be considered to be either a complication to or follow-up for the index fracture and thus not be a cause for inclusion in the respective fracture cohort. Hence, patients can contribute to the treatment gap analysis in several fracture cohorts, yet only once per fracture group (MOF, hip, vertebral, humerus, forearm) per calendar year, with the rationale being that the same patient can present a missed opportunity for anti-osteoporosis treatment in more than one calendar year and for more than one type of fracture. Patients were excluded if they had a history of breast or prostate cancer, bone metastasis, and/or Paget’s disease.
The diagnosis codes used to identify fractures are listed in supplementary table 1.
Data extraction
For each participant in each cohort, the date of the index fracture was defined as baseline. The baseline period to identify demographic and clinical characteristics was defined as the year prior to the index fracture for use of pharmaceutical products, the last 5 years prior to the index fracture for Charlson Comorbidity index, and any time prior to baseline for other baseline covariates including medical history.
Follow-up data included treatment with AOM from baseline until censored, defined by treatment with bisphosphonates (including zoledronic acid), raloxifene, teriparatide, denosumab, or strontium ranelate. Anti-osteoporosis medication therapy was identified as the filling of at least one prescription at a pharmacy, and/or by at least one procedure code (SKS code, which is a Danish healthcare classification system) for administration of zoledronic acid or denosumab. Users of AOM who filled prescriptions but also received hospital-administered AOM (by “SKS” code) were counted only in the hospital-administered AOM group. The ATC and SKS codes used to identify AOM are listed in supplementary table 1.
Treatment with glucocorticoids (only oral glucocorticoids were included) and hormone replacement therapy was defined by the filling of at least one prescription at a pharmacy.
Statistical analysis
Each annual fracture cohort was analyzed separately. As part of the repeated cross-sectional design, patients entered each cohort at the time of their first eligible incident fracture in the respective calendar year. Patients were censored at the time of death, migration, or 1 year after the index fracture, whichever occurred first.
Treatment persistence was operationalized as either the filling of a receipt for an AOM within 120 days of the 1-year anniversary for the first AOM treatment administration following the index fracture (as a pack of alendronic acid lasts up to 84 days, and then adding a grace period of 25%) or as filled prescriptions (in Defined Daily Doses) accounting for at least one full year of AOM use (including a 25% grace period). Patients receiving zoledronic acid infusion were considered treatment persistent at 1-year.
To identify factors associated with AOM treatment initiation, we performed a logistic regression analysis for the 2014 MOF cohort, with all baseline covariates used as input into the model.
Data were analyzed using Stata version 16.1. Categorical data were summarized by number and proportion of patients, while continuous variables were summarized by mean. All analyses were stratified according to the year and type of fracture. Outcomes analyses were performed on an overall level, and also stratified according to gender and age at index fracture (<75 vs ≥75 years).
Results
Demographics of the fracture populations
For the MOF cohorts, a total of 249,897 fracture events met the criteria for evaluation for the study. Following exclusion of patients with a diagnosis code for breast cancer (n = 10,133), prostate cancer (n = 3192), or bone metastasis or Paget’s disease (n = 392), a total of 236,180 fracture events were included in the MOF cohorts (Table 1). For the hip, vertebral, humerus, and forearm fracture cohorts, a total of 81,498, 18,343, 46,303, and 98,602 fracture events were included, respectively (supplementary tables 2 to 5). The number of hip fractures decreased over the years, while the number of vertebral fractures increased.
The mean age of the MOF cohorts were 72.6–73.9 years and 24.3–26.6% were men (Table 2). A major osteoporotic fracture had previously been sustained in 24–29% of the MOF patients, while 33–40% had previously sustained any fracture. Less than 10% had been treated with corticosteroids in the previous year.
Compared to the MOF cohorts, the hip fracture cohorts (supplementary table 6) were older at baseline (mean age around 80 years), and more were men (28–31%). A larger proportion had previously sustained a major osteoporotic fracture (30–35%) or any fracture (39–45%), and 12–13% had previously sustained a hip fracture. The vertebral, humerus, and forearm fracture cohorts (supplementary tables 7 to 9) were overall comparable to the MOF cohorts, except that the vertebral fracture cohorts had higher proportions of men (40–44%), while the forearm fracture cohorts were younger (mean age around 70 years) and had lower proportions of men (18–19%).
Treatment gap
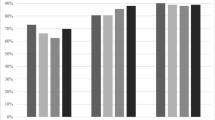
The post-fracture treatment gap in the 2005 MOF cohort was approximately 85%, decreasing to 79% in 2014 (Fig. 1). The narrowing treatment gap was mainly due to more patients already on anti-osteoporosis therapy at the time of fracture, increasing from 8% in 2005 to 12% in 2014, although demonstrating a decreasing trend from 2012 to 2014. The proportion of fracture patients initiating treatment within 1 year of their fracture increased from 7% to 9%. While most treatment initiations were prescription-based, an increasing proportion of fracture patients were initiated on hospital-administered AOM. In 2014, however, this was still as little as 0.6% of all patients in the MOF cohort.
Treatment persistence, defined as patients receiving AOM within 1 year of the index fracture and still receiving AOM after an additional year, was found to be 81% across the annualized fracture cohorts.
Treatment gap according to fracture location
Stratifying the treatment gap analysis according to fracture location (Fig. 2), we found that the treatment gaps for hip (panel 2A) and forearm (panel 2D) fracture patients decreased over the time span of our analysis, from a starting point in 2005 at 82% and 88%, respectively, to 74% and 82% in 2014. This was driven by an increase in the proportion of patients already on AOM therapy at the time of the fracture and remaining on therapy post-fracture, and—primarily—for hip fracture patients also by an increase in the proportion of patients being initiated on AOM therapy after the fracture.
Treatment rate and treatment gap for patients sustaining a first incident hip (panel 2A), vertebral (2B), humerus (2C), or forearm (2D) fracture within any given year (2005–2014). The numbers above the bars indicate the proportion of patients initiating hospital-administered treatment (zoledronic acid or denosumab); no number listed if too few observations
In comparison, the treatment gaps for patients with vertebral (panel 2B) and humerus (panel 2C) fractures were relatively stable from 2005 to 2014. Patients with vertebral fractures were significantly more likely to receive AOM treatment within 1 year of the index fracture (treatment gap of 63 to 66%), driven both by more patients already on treatment at the time of the fracture and by more patients initiating treatment after the fracture. The treatment gap in humerus fracture patients varied between 83 and 88%.
Initiation of hospital-administered AOM increased for all fracture types from 2005 to 2014, yet were more frequently initiated in patients with vertebral (0.8 to 2.2%) and hip (0.1 to 1.3%) fractures, as compared to patients with humerus (0 to 0.6%) and forearm (0 to 0.4%) fractures. For all fracture types, initiation of hospital-administered AOM seemed to stagnate or even decrease from 2012 and onwards.
Treatment gap according to gender
The treatment gap was significantly smaller in women than in men, irrespective of index fracture cohort and index year (Fig. 3 and supplementary figures 1–4). In men, the treatment gap in the MOF and hip fracture cohorts decreased from 2005 to 2014, while it was stable in the vertebral, humerus, and forearm fracture cohorts. In women, the treatment gap decreased from 2005 to 2014, irrespective of fracture cohort.
Treatment rate and treatment gap for patients sustaining a first incident major osteoporotic fracture within any given year (2005–2014), stratified according to gender. The numbers above the bars indicate the proportion of patients initiating hospital-administered treatment (zoledronic acid or denosumab). Treatment gap lines are trend lines
Treatment gap according to age at index fracture
The treatment gap was consistently smaller in older (age ≥ 75 years at index fracture) than in younger (<75 years) patients, except for hip fracture patients in whom the treatment gap was similar regardless of the age stratification (supplementary figures 5–9). In the MOF, humerus, and forearm fracture cohorts the differences between the younger vs the older age group was generally below 10%, whereas in the vertebral fracture cohort the differences between the groups fluctuated around 15%. During the time span of our analysis, the treatment gap demonstrated a decreasing trend in both age groups across the fracture cohorts, except for vertebral fracture patients in whom it was relatively stable.
Proportion of AOM not captured in prescription registers
The amount of hospital-administered AOM out of the total uptake of osteoporosis medications was relatively small over the period studied. Specifically, the use of hospital-administered AOM as a proportion of total AOM use within 1 year after a major osteoporotic fracture increased initially, yet appears to decrease towards the end of our analysis (Fig. 4). During our analysis, an average of 3.9% of the AOM-treated MOF patients where using hospital-administered AOM. The general trend was that hospital-administered AOM were relatively more often used in men, and use of hospital-administered AOM varied significantly between fracture cohorts and index fracture year, ranging from 0 to 18% (supplementary table 10).
Use of hospital-administered AOM in patients sustaining a first incident major osteoporotic fracture in any given year (2005–2014). The figure shows the proportion of all AOM-treated MOF patients treated with hospital-administered AOM (zoledronic acid or denosumab), stratified according to gender. Index year has been grouped for smoothening. AOM, anti-osteoporosis medication; MOF, major osteoporotic fracture
Factors associated with AOM treatment
In the 2014 MOF cohort, age (odds ratio [OR] 1.01; 95% confidence interval [CI] 1.00–1.01), treatment with corticosteroids (OR 2.32; 95% CI 2.10–2.59), and hormone replacement therapy (OR 1.22; 95% CI 1.11–1.35) were associated with an increased likelihood of receiving AOM treatment. Male gender was associated with a reduced likelihood of treatment (OR 0.37; 95% CI 0.34–0.40). Patients with a prior MOF (OR 1.38; 95% CI 1.22–1.54) or any prior fracture (OR 1.32; 95% CI 1.19–1.48) were more likely to receive treatment.
As compared to patients with an index forearm fracture, patients with an index vertebral fracture (OR 3.00; 95% CI 2.69–3.35) or an index hip fracture (OR 1.60; 95% CI 1.48–1.73) were more likely to receive AOM treatment, whereas index humerus fractures where associated with a reduced likelihood of treatment (OR 0.84; 95% CI 0.76–0.94).
Using a CCI score of 0 as the reference, a CCI of 1 was associated with an increased likelihood of receiving AOM treatment (OR 1.22; 95% CI 1.09–1.36), a CCI of 2 was not different from the reference at a statistically significant level (OR 1.11; 95% CI 0.94–1.30), and a CCI of 3 or above was associated with a reduced likelihood of receiving AOM treatment (OR 0.79; 95% CI 0.64–0.96).
Discussion
Our findings demonstrate a critical gap in post-fracture care with large and persistent anti-osteoporosis treatment gaps in men and women sustaining a MOF. For the hip and forearm fracture cohorts, the treatment gap—based on the filling of at least one prescription or receiving at least one parenteral osteoporosis drug dose—decreased marginally from 2005 to 2014, yet in the 2014 cohorts, 3 out of 4 hip fracture patients and 4 out of 5 forearm fracture patients were still not treated with AOM within 1 year of the index fracture. The treatment gaps for vertebral and humerus fracture patients were generally stable at around 65% and 85%, respectively, across the 2005 to 2014 time period. The treatment gap was more pronounced in men than in women, and in younger than in older patients (<75 vs ≥75 years). It should be noted that not all fragility fracture patients are candidates for AOM treatment [31], as contraindications, patient preferences, and other individual or local factors may speak against treatment initiation. Furthermore, Danish treatment guidelines recommend that AOM treatment decisions in patients with non-hip non-vertebral fragility fractures should be based also on further evaluations to estimate the risk of subsequent fractures [11, 12], which may be reflected in the treatment gap among humerus and forearm fracture patients identified in this study. However, in order to benchmark the performance of the Danish hospital services against international metrics we use the term treatment gap in the internationally accepted sense. Also, it is worth noting that FRAX based guidelines largely set the intervention threshold at the equivalence point of a postmenopausal woman with a prior fragility fracture [32]. Our findings demonstrate that the use of AOM in secondary fracture prevention is currently insufficient, which is in line with what has been demonstrated in previous studies. In MOF patients not treated with AOM at the time of the fracture, Leslie et al. (2012) found a persistent treatment gap above 85% in Canada between 1996 and 2008, with the gap being larger in men [25]. Also including patients treated with AOM at the time of the fracture, Wilk et al. (2014) demonstrated a 1 year post-fracture treatment gap around 75% in women with hip fractures in the USA [21], consistent with a treatment gap around 73% in men and women with hip fractures in Hawaii as demonstrated by Nguyen et al. (2018) [26]. Other studies have demonstrated similar findings [17, 19], indicating that the lack of post-fracture anti-osteoporosis medical therapy seems to be a universal problem. Barton and colleagues (2019) demonstrated decreasing treatment rates in AOM naïve patients sustaining an incident vertebral fracture, suggesting that this may be due to fear of rare bisphosphonate side effects such as osteonecrosis of the jaw and atypical femur fractures [33]. However, while some time trend analyses indicate that the problem may be getting worse in recent years [19, 25, 29], our analysis suggests a stable or slightly improving treatment gap from 2005 to 2014 in Denmark.
The post-fracture treatment gap in Denmark was evaluated recently—conjointly with the United Kingdom and Catalonia—using comparable data sources linked to the IFRISK study, demonstrating large and persistent treatment gaps in Denmark [27]. This current paper was conceptualized to evaluate if inclusion of hospital-administered AOM would reduce this treatment gap. Further, we here evaluate the treatment gap after any MOF allowing each individual to contribute to several fracture cohorts representing additional missed opportunities for AOM treatment initiation, whereas the first paper evaluated the treatment gap after any first incident fragility fracture. Reassuringly, this current paper demonstrates smaller—yet still very large—treatment gaps in Denmark in patients with a major osteoporotic fracture as compared to any osteoporotic fracture as was reported previously. For hip, vertebral, and forearm fractures (MOF and humerus fractures were not independently evaluated in the former paper) the treatment gaps are generally smaller in this current analysis, probably owing to the differences in study design and the inclusion of hospital-administered AOM [27].
The cause of the treatment gap could plausibly, at least in part, be attributed to insufficient coordination of care. Several structural models to improve post-fracture care have been developed, and a review evaluating 10 such interventions reported all of them demonstrating improvements in post-fracture initiation of AOM therapy [34]. Among these care models, the fracture liaison service (FLS) has, in particular, been demonstrated to effectively improve treatment initiation rates and treatment compliance in a cost-effective manner [35, 36]. However, while the use of FLS units is being employed across the globe and a best practice framework has been defined, no accredited FLS units were in place in Denmark during the time span of our analysis, and today only four Danish hospitals run an internationally accredited FLS (www.capturethefracture.org) [36]. The lack of a coordinated effort to initiate evaluation and treatment in fracture patients almost certainly contributed to the high rates of untreated MOF patients seen in our analysis. Another reason for the treatment gap may include a lack of knowledge in patients and healthcare providers about the risks and benefits of AOM, as suggested elsewhere [29, 37].
In this analysis, we also found that inclusion of hospital-administered AOM in the treatment gap assessment did not materially reduce the treatment gap after a MOF in Denmark. Hence, an average of only 3.9% of AOM-treated patients in the MOF cohorts were using hospital-administered AOM (zoledronic acid or denosumab) within 1 year of their index fracture. This demonstrated substantial variation between genders and index fracture types, with proportionately more men than women using hospital-administered AOM, and with hospital-administered AOM being used more often in vertebral fracture patients (supplementary table 10). Hospital-administered AOM was initiated more often in vertebral and hip fracture patients than in humerus and forearm fracture patients (Fig. 2).
In a recent study, Axelsson et al. (2020) attempted to identify the use of hospital-administered AOM by combining a diagnosis code for osteoporosis with codes for intravenous or subcutaneous drug administration, using Swedish health registers. While the validity of this approach is uncertain, they found an increasing proportion of fracture patients meeting these criteria for parenteral AOM-administration following implementation of an FLS unit (going from 11.8 to 18.9%) [28]. Cheung et al. (2018) reported in an analysis of the Hong Kong AOM treatment gap, that of all patients on AOM, 4% where on zoledronic acid and 1% on denosumab [19]. Solomon et al. (2014) reported that 0.3% of AOM-treated patients were using denosumab within 1 year of the index fracture, yet did not provide distinct figures on the use of zoledronic acid [29]. Including hospital-administered AOM in the treatment gap assessment does not seem to materially alter the treatment rate in Denmark, yet this may differ significantly between countries. In this regard, it is important to note that zoledronic acid was first granted marketing authorization for use in osteoporosis by the European Medicines Agency in 2005, and denosumab in 2010. Thus, the years covered in this study reflects the early use of both drugs, which may partially explain our findings. Hence, future treatment gap evaluations should preferably include hospital-administered AOM.
The reason for vertebral and hip fractures more commonly being a cause for initiating hospital-administered AOM than humerus and forearm fractures, could be that Danish osteoporosis guidelines recognize the former as diagnostic for osteoporosis [11, 12] in line with these fractures carrying a very high risk of subsequent fractures [38]. This could potentially instill a greater willingness among the treating physicians to use hospital resources to ensure AOM treatment insofar contraindications or side effects to oral therapies exist. The significant reduction in mortality in hip fracture patients treated with zoledronic acid, as demonstrated in the HORIZON Recurrent Fracture Trial, may also have contributed to this group more frequently being prescribed a hospital-administered AOM [39].
The notion of a higher proportion of men than women being treated with hospital-administered AOM may reflect the possibility that side effects and/or contraindications to oral AOM could occur more frequently in men. Further, hospital administration of AOM may be less prone to primary non-compliance; hence, the finding may reflect a higher proportion of men than women being prescribed a hospital-administered therapy due to a perceived risk of primary non-compliance at the time of AOM-prescription.
Based on the logistic regression analysis for patients sustaining a MOF (in 2014), we reassuringly found that patients with an index vertebral or hip fracture were more likely to receive AOM treatment, which is in line with the Danish treatment guidelines recognizing these fractures as diagnostic for osteoporosis [11, 12]. We also found an apparent association between common fracture risk factors (including age, prior fractures and corticosteroid treatment) and AOM treatment, indicating that patients who would seem to be at higher risk of subsequent fractures are indeed more likely to receive AOM treatment. Treatment was less likely in subjects with a low comorbidity score (Charlson index of zero) but also in patients with substantial comorbidity (Charlson index of 3 or higher), the latter likely results from a combination of contraindications against bisphosphonates or a clinical decision based on expected short remaining lifespan. We also confirmed that male gender was associated with a markedly reduced propensity to receive AOM treatment, indicating that male osteoporosis is still neglected in clinical practice. These findings could be used to tailor future secondary fracture prevention strategies.
The strengths of this study include the use of validated health registries and prescription databases, and the large number of patients and fractures accrued over a substantial time period without data breaks. Further, to our knowledge this is the first study using Danish data to include hospital-administered AOM therapy in the evaluation of the post-fracture AOM treatment gap.
Limitations do exist, including the small risk of diagnosis miscoding which is an inherent part of registry-based research. Further, prescription data originates at pharmacy level, thus reflecting filled prescriptions. Hence, we are not able to ascertain the number of patients actually prescribed an AOM. Further, we cannot assess from our data if patients filling their prescriptions actually ingest their AOM. This, coupled with the fact that our study did not require patients to remain on AOM throughout the full year post-fracture, likely resulted in an underestimation of the true treatment gap. To this end, we found a 1-year treatment persistence around 81%.
An additional limitation pertains to the use of zoledronic acid. From the data, we cannot ascertain if it has been administered due to osteoporosis or in the oncology-setting (e.g. tumor-induced hypercalcaemia). Hence, in this study the indication for using zoledronic acid following an index fracture may be biased, yet even so, fracture patients receiving zoledronic acid—regardless of indication—does not contribute to the treatment gap, why this would merely serve to deflate the treatment gap and thus does not affect the conclusions of our study.
Another aspect is the completeness of our data in terms of hospital administration of zoledronic acid and denosumab by the use of procedure codes. While this has not been formally validated, there is a sizeable yet redeemable cost to the department for such hospital administration; hence, we are confident that the vast majority of administrations were indeed captured.
In conclusion, this analysis has demonstrated that a major gap in post-fracture AOM treatment was evident in patients sustaining a major osteoporotic fracture in Denmark from 2005 to 2014. Regrettably, the magnitude of this gap was not materially altered by the inclusion of hospital-administered AOM. While our results echo the call for strengthening post-fracture care, this study also highlights the continued need for a systematic, proactive, nationwide approach to the organization of secondary fracture prevention.
Data availability
Not available due to legislative restrictions on data sharing.
Code availability
Not available.
References
Brandi ML (2009) Microarchitecture, the key to bone quality. Rheumatol (United Kingdom) 48:iv3–iv8. https://doi.org/10.1093/rheumatology/kep273
Hernlund E, Svedbom A, Ivergård M et al (2013) Osteoporosis in the European Union: Medical management, epidemiology and economic burden: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 8:1–115. https://doi.org/10.1007/s11657-013-0136-1
Driessen JHM, Hansen L, Eriksen SA et al (2016) The epidemiology of fractures in Denmark in 2011. Osteoporos Int 27:2017–2025. https://doi.org/10.1007/s00198-016-3488-8
Klotzbuecher CM, Ross PD, Landsman PB et al (2000) Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 15:721–739. https://doi.org/10.1359/jbmr.2000.15.4.721
Van Geel T, Van Helden S, Geusens P et al (2009) Clinical subsequent fractures cluster in time after first fractures. Ann Rheum Dis 68:99–102. https://doi.org/10.1136/ard.2008.092775
Johansson H, Siggeirsdóttir K, Harvey NC et al (2017) Imminent risk of fracture after fracture. Osteoporos Int 28:775–780. https://doi.org/10.1007/s00198-016-3868-0
Port L, Center J, Briffa NK et al (2003) Osteoporotic fracture: missed opportunity for intervention. Osteoporos Int 14:780–784. https://doi.org/10.1007/s00198-003-1452-x
Edwards BJ, Bunta AD, Simonelli C et al (2007) Prior fractures are common in patients with subsequent hip fractures. Clin Orthop Relat Res:226–230. https://doi.org/10.1097/BLO.0b013e3180534269
Frederiksen A, Abrahamsen B, Johansen PB, Sorensen HA (2018) Danish, national cross-sectional observational study on the prevalence of prior major osteoporotic fractures in adults presenting with hip fracture-limitations and scope for fracture liaison services in prevention of hip fracture. Osteoporos Int 29:109–114. https://doi.org/10.1007/s00198-017-4247-1
Geusens P, Lems WF, Bours S, vd Bergh JP (2019) Secondary fracture prevention: drug treatment, fall prevention and nutrition requirements. Best Pract Res Clin Rheumatol 33:290–300. https://doi.org/10.1016/j.berh.2019.04.005
Hitz M, Harsløf T, Ejersted C, et al (2019) NBV: postmenopausal osteoporose
Hermann P, Frost M, Abrahamsen B, et al (2020) NBV: Behandling af mandlig osteoporose
Kanis J, Cooper C, Rizzoli R et al (2019) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 30:3–44. https://doi.org/10.1007/s00198-018-4704-5
Cooper C, Ferrari S, IOF Board and Executive Committee (2017) IOF Compendium of Osteoporosis
Compston J, Cooper A, Cooper C et al (2017) UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos 12:43. https://doi.org/10.1007/s11657-017-0324-5
Conley RB, Adib G, Adler RA et al (2020) Secondary fracture prevention: consensus clinical recommendations from a multistakeholder coalition. J Bone Miner Res 35:36–52. https://doi.org/10.1002/jbmr.3877
Elliot-Gibson V, Bogoch ER, Jamal SA, Beaton DE (2004) Practice patterns in the diagnosis and treatment of osteoporosis after a fragility fracture: a systematic review. Osteoporos Int 15:767–778. https://doi.org/10.1007/s00198-004-1675-5
Papaioannou A, Giangregorio L, Kvern B et al (2004) The osteoporosis care gap in Canada. BMC Musculoskelet Disord 5:1–6. https://doi.org/10.1186/1471-2474-5-11
Cheung M, Ho AW, Wong S (2018) Post-fracture care gap: a retrospective population-based analysis of Hong Kong from 2009 to 2012. Hong Kong Med J 24:579–583. https://doi.org/10.12809/hkmj187227
Fraser LA, Ioannidis G, Adachi JD et al (2011) Fragility fractures and the osteoporosis care gap in women: the Canadian Multicentre Osteoporosis Study. Osteoporos Int 22:789–796. https://doi.org/10.1007/s00198-010-1359-2
Wilk A, Sajjan S, Modi A et al (2014) Post-fracture pharmacotherapy for women with osteoporotic fracture: analysis of a managed care population in the USA. Osteoporos Int 25:2777–2786. https://doi.org/10.1007/s00198-014-2827-x
Shibli-Rahhal A, Vaughan-Sarrazin MS, Richardson K, Cram P (2011) Testing and treatment for osteoporosis following hip fracture in an integrated U. S. healthcare delivery system. Osteoporos Int 22:2973–2980. https://doi.org/10.1007/s00198-011-1536-y
Papaioannou A, Kennedy CC, Ioannidis G et al (2008) The osteoporosis care gap in men with fragility fractures: the Canadian Multicentre Osteoporosis Study. Osteoporos Int 19:581–587. https://doi.org/10.1007/s00198-007-0483-0
Lüthje P, Nurmi-Lüthje I, Kaukonen JP et al (2009) Undertreatment of osteoporosis following hip fracture in the elderly. Arch Gerontol Geriatr 49:153–157. https://doi.org/10.1016/j.archger.2008.06.007
Leslie WD, Giangregorio LM, Yogendran M et al (2012) A population-based analysis of the post-fracture care gap 1996-2008: the situation is not improving. Osteoporos Int 23:1623–1629. https://doi.org/10.1007/s00198-011-1630-1
Nguyen ET, Posas-Mendoza T, Siu AM et al (2018) Low rates of osteoporosis treatment after hospitalization for hip fracture in Hawaii. Osteoporos Int 29:1827–1832. https://doi.org/10.1007/s00198-018-4553-2
Skjødt MK, Khalid S, Ernst M et al (2020) Secular trends in the initiation of therapy in secondary fracture prevention in Europe: a multi-national cohort study including data from Denmark, Catalonia, and the United Kingdom. Osteoporos Int 31:1535–1544. https://doi.org/10.1007/s00198-020-05358-4
Axelsson KF, Johansson H, Lundh D et al (2020) Association Between recurrent fracture risk and implementation of fracture liaison services in four swedish hospitals: a cohort study. J Bone Miner Res 35:1216–1223. https://doi.org/10.1002/jbmr.3990
Solomon DH, Johnston SS, Boytsov NN et al (2014) Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J Bone Miner Res 29:1929–1937. https://doi.org/10.1002/jbmr.2202
(2018) Multinational Observational Database Study on Imminent Osteoporotic Fracture Risk: Stage 1. In: Eur. Union Electron. Regist. Post-Authorisation Stud. (EU PAS Regist. http://www.encepp.eu/encepp/viewResource.htm?id=24100. Accessed 23 Jun 2020
Javaid MK, Sami A, Lems W et al (2020) A patient-level key performance indicator set to measure the effectiveness of fracture liaison services and guide quality improvement: a position paper of the IOF Capture the Fracture Working Group, National Osteoporosis Foundation and Fragility Fracture. Osteoporos Int 31:1193–1204. https://doi.org/10.1007/s00198-020-05377-1
Kanis JA, Harvey NC, Johansson H et al (2017) FRAX update. J Clin Densitom 20:360–367. https://doi.org/10.1016/j.jocd.2017.06.022
Barton DW, Behrend CJ, Carmouche JJ (2019) Rates of osteoporosis screening and treatment following vertebral fracture. Spine J 19:411–417. https://doi.org/10.1016/j.spinee.2018.08.004
Little EA, Eccles MP (2010) A systematic review of the effectiveness of interventions to improve post-fracture investigation and management of patients at risk of osteoporosis. Implement Sci 5. https://doi.org/10.1186/1748-5908-5-80
Ganda K, Puech M, Chen J et al (2013) Models of care for the secondary prevention of osteoporotic fractures: a systematic review and meta-analysis. Osteoporos Int 24:393–406. https://doi.org/10.1007/s00198-012-2090-y
Mitchell P, Åkesson K, Chandran M et al (2016) Implementation of Models of Care for secondary osteoporotic fracture prevention and orthogeriatric Models of Care for osteoporotic hip fracture. Best Pract Res Clin Rheumatol 30:536–558. https://doi.org/10.1016/j.berh.2016.09.008
Jaglal SB, McIsaac WJ, Hawker G et al (2003) Information needs in the management of osteoporosis in family practice: an illustration of the failure of the current guideline implementation process. Osteoporos Int 14:672–676. https://doi.org/10.1007/s00198-003-1421-4
Center JR, Bliuc D, Nguyen TV, Eisman JA (2007) Risk of subsequent fracture after low-trauma fracture in men and women. JAMA 297:387–394. https://doi.org/10.1001/jama.297.4.387
Lyles KW, Colón-Emeric CS, Magaziner JS et al (2007) Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 357:1799–1809. https://doi.org/10.1056/NEJMoa074941
Funding
UCB funded this study. All analyses were conducted independently by the academic researchers involved.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Danish Medicines Agency, the Danish Data Protection Agency, and Statistics Denmark (ref. number 706638). For this type of studies, ethical committee approval is not required. For this type of study formal consent is not required.
Consent to participate
Not applicable.
Consent for publication
Given by all authors.
Conflict of interest
MKS: Educational grant and institutional research grant, UCB.
MTE: Institutional grant, UCB.
SK: No conflict of interest.
CL and ET: Employees of UCB pharma.
CC: Personal fees from Alliance for Better Bone Health, Amgen, Eli Lilly, GSK, Medtronic, Merck, Novartis, Pfizer, Roche, Servier, Takeda and UCB.
AD: No conflict of interest.
KHR: No conflict of interest.
MKJ: Personal fees from Amgen and UCB, unrestricted grants from Amgen.
DML: Consulting or speakers fees from Amgen, Lilly, Novartis, Italfarmaco, Ferrer and Rubió, outside the submitted work.
DPA: Research grants from Amgen, Johnson & Johnson, and UCB. Consultation or speaker fees paid to my department by Amgen, Astellas, and UCB.
BA: Institutional research grants UCB, Novartis. Consulting or speakers fees UCB, Kyowa-Kirin, Amgen, Eli-Lily, Pharmacosmos.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 47626 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Skjødt, M.K., Ernst, M.T., Khalid, S. et al. The treatment gap after major osteoporotic fractures in Denmark 2005-2014: a combined analysis including both prescription-based and hospital-administered anti-osteoporosis medications. Osteoporos Int 32, 1961–1971 (2021). https://doi.org/10.1007/s00198-021-05890-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-021-05890-x