Abstract
Summary
Osteoporosis is a major concern in patients with Duchenne muscular dystrophy. In this novel study of teriparatide treatment in 6 patients with severe osteoporosis, bone health (fractures, vertebral morphometry, and DXA) remained stable, with no adverse events. These findings will help inform future osteoporosis research in this challenging population.
Introduction
Despite standard therapy with vitamin D and bisphosphonates (BP), many patients with Duchenne muscular dystrophy (DMD) continue to sustain fragility fractures due to long-term glucocorticoid treatment and limited mobility. We aimed to evaluate the safety and efficacy of teriparatide for the treatment of severe osteoporosis in adolescent and young adult patients with DMD.
Methods
We prospectively treated 6 patients with DMD who had severe osteoporosis with teriparatide 20 mcg subcutaneously daily for 1–2 years. Inclusion criteria were long-term glucocorticoid therapy, and severe osteoporosis despite treatment with BP, or intolerance to BP. We examined long bone and vertebral fracture outcomes, including vertebral morphometry measures, bone mineral density and content, bone formation markers, safety indices, and adverse events.
Results
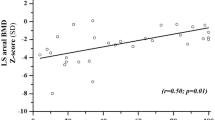
The mean age at teriparatide start was 17.9 years (range 13.9–22.1 years). All 6 patients were on daily glucocorticoids (mean ± SD; duration 10.9 ± 2.5 years) and 5 were non-ambulatory. Five patients had been treated with BP for 7.9 ± 4.2 years. All had vertebral and a history of long bone fragility fractures at baseline. Vertebral heights and Genant fracture grading remained stable. Long bone fracture rate appeared to decrease (from 0.84/year to 0.09/year); one patient sustained a long bone fracture at 6 months of treatment. Trajectories for change in bone mineral density and content were not different post- vs. pre-teriparatide. Procollagen type 1 amino-terminal propeptide (P1NP) increased, while laboratory safety indices remained stable and non-concerning. No adverse events were observed.
Conclusion
In six patients with DMD treated with teriparatide for severe osteoporosis, we observed stable bone health and modest increases in P1NP, without safety concerns. Further studies are needed to better understand teriparatide efficacy for treatment of osteoporosis in patients with DMD.



Similar content being viewed by others
Abbreviations
- aBMD:
-
Areal bone mineral density
- BMAD:
-
Bone mineral apparent density
- BMD:
-
Bone mineral density
- BMC:
-
Bone mineral content
- BP:
-
Bisphosphonate(s)
- DMD:
-
Duchenne muscular dystrophy
- DXA:
-
Dual X-ray absorptiometry
- eGFR:
-
Estimated glomerular filtration rate
- GC:
-
Glucocorticoid(s)
- GIO:
-
Glucocorticoid-induced osteoporosis
- LDF:
-
Lateral distal femur
- LS:
-
Lumbar spine
- P1NP:
-
Procollagen type 1 amino-terminal propeptide
- PTH:
-
Parathyroid hormone
- rhPTH:
-
Recombinant human PTH
References
Bianchi ML, Biggar D, Bushby K, Rogol AD, Rutter MM, Tseng B (2011) Endocrine aspects of Duchenne muscular dystrophy. Neuromuscul Disord 21(4):298–303. https://doi.org/10.1016/j.nmd.2011.02.006
Mayo AL, Craven BC, McAdam LC, Biggar WD (2012) Bone health in boys with Duchenne muscular dystrophy on long-term daily deflazacort therapy. Neuromuscul Disord 22:1040–1045. https://doi.org/10.1016/j.nmd.2012.06.354
Birnkrant DJ, Bushby K, Bann CM, Apkon SD, Blackwell A, Brumbaugh D, Case LE, Clemens PR, Hadjiyannakis S, Pandya S, Street N, Tomezsko J, Wagner KR, Ward LM, Weber DR, Group DMDCCW (2018) Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol 17(3):251–267. https://doi.org/10.1016/S1474-4422(18)30024-3
Birnkrant DJ, Bushby K, Bann CM, Alman BA, Apkon SD, Blackwell A, Case LE, Cripe L, Hadjiyannakis S, Olson AK, Sheehan DW, Bolen J, Weber DR, Ward LM, Group DMDCCW (2018) Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol 17(4):347–361. https://doi.org/10.1016/S1474-4422(18)30025-5
Larson CM, Henderson RC (2000) Bone mineral density and fractures in boys with Duchenne muscular dystrophy. J Pediatr Orthop 20(1):71–74
Ma J, McMillan HJ, Karaguzel G, Goodin C, Wasson J, Matzinger MA, DesClouds P, Cram D, Page M, Konji VN, Lentle B, Ward LM (2017) The time to and determinants of first fractures in boys with Duchenne muscular dystrophy. Osteoporosis Int 28(2):597–608. https://doi.org/10.1007/s00198-016-3774-5
Ward LM, Hadjiyannakis S, McMillan HJ, Noritz G, Weber DR (2018) Bone health and osteoporosis management of the patient with Duchenne muscular dystrophy. Pediatrics 142(Suppl 2):S34–S42. https://doi.org/10.1542/peds.2018-0333E
Hawker GA, Ridout R, Harris VA, Chase CC, Fielding LJ, Biggar WD (2005) Alendronate in the treatment of low bone mass in steroid-treated boys with Duchennes muscular dystrophy. Arch Phys Med Rehabil 86(2):284–288. https://doi.org/10.1016/j.apmr.2004.04.021
Sbrocchi AM, Rauch F, Jacob P, McCormick A, McMillan HJ, Matzinger MA, Ward LM (2012) The use of intravenous bisphosphonate therapy to treat vertebral fractures due to osteoporosis among boys with Duchenne muscular dystrophy. Osteoporosis Int 23:2703–2711. https://doi.org/10.1007/s00198-012-1911-3
Srinivasan R, Rawlings D, Wood CL, Cheetham T, Moreno AC, Mayhew A, Eagle M, Guglieri M, Straub V, Owen C, Bushby K, Sarkozy A (2016) Prophylactic oral bisphosphonate therapy in duchenne muscular dystrophy. Muscle Nerve 54(1):79–85. https://doi.org/10.1002/mus.24991
de Nijs RN, Jacobs JW, Algra A, Lems WF, Bijlsma JW (2004) Prevention and treatment of glucocorticoid-induced osteoporosis with active vitamin D3 analogues: a review with meta-analysis of randomized controlled trials including organ transplantation studies. Osteoporosis Int 15(8):589–602. https://doi.org/10.1007/s00198-004-1614-5
Buckley L, Guyatt G, Fink HA, Cannon M, Grossman J, Hansen KE, Humphrey MB, Lane NE, Magrey M, Miller M, Morrison L, Rao M, Robinson AB, Saha S, Wolver S, Bannuru RR, Vaysbrot E, Osani M, Turgunbaev M, Miller AS, McAlindon T (2017) 2017 American College of Rheumatology Guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol 69(8):1521–1537. https://doi.org/10.1002/art.40137
Buckley L, Humphrey MB (2018) Glucocorticoid-induced osteoporosis. N Engl J Med 379(26):2547–2556. https://doi.org/10.1056/NEJMcp1800214
Brixen KT, Christensen PM, Ejersted C, Langdahl BL (2004) Teriparatide (biosynthetic human parathyroid hormone 1-34): a new paradigm in the treatment of osteoporosis. Basic Clin Pharmacol Toxicol 94(6):260–270. https://doi.org/10.1111/j.1742-7843.2004.pto940602.x
Saag KG, Shane E, Boonen S, Marin F, Donley DW, Taylor KA, Dalsky GP, Marcus R (2007) Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med 357(20):2028–2039. https://doi.org/10.1056/NEJMoa071408
Cipriani C, Irani D, Bilezikian JP (2012) Safety of osteoanabolic therapy: a decade of experience. J Bone Miner Res 27(12):2419–2428. https://doi.org/10.1002/jbmr.1800
Orwoll ES, Scheele WH, Paul S, Adami S, Syversen U, Diez-Perez A, Kaufman JM, Clancy AD, Gaich GA (2003) The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone Miner Res 18(1):9–17. https://doi.org/10.1359/jbmr.2003.18.1.9
Nakamura T, Sugimoto T, Nakano T, Kishimoto H, Ito M, Fukunaga M, Hagino H, Sone T, Yoshikawa H, Nishizawa Y, Fujita T, Shiraki M (2012) Randomized Teriparatide [human parathyroid hormone (PTH) 1-34] Once-Weekly Efficacy Research (TOWER) trial for examining the reduction in new vertebral fractures in subjects with primary osteoporosis and high fracture risk. J Clin Endocrinol Metab 97(9):3097–3106. https://doi.org/10.1210/jc.2011-3479
Saag KG, Zanchetta JR, Devogelaer JP, Adler RA, Eastell R, See K, Krege JH, Krohn K, Warner MR (2009) Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: thirty-six-month results of a randomized, double-blind, controlled trial. Arthritis Rheum 60(11):3346–3355. https://doi.org/10.1002/art.24879
Winer KK, Zhang B, Shrader JA, Peterson D, Smith M, Albert PS, Cutler GB Jr (2012) Synthetic human parathyroid hormone 1-34 replacement therapy: a randomized crossover trial comparing pump versus injections in the treatment of chronic hypoparathyroidism. J Clin Endocrinol Metab 97(2):391–399. https://doi.org/10.1210/jc.2011-1908
Winer KK (2019) Advances in the treatment of hypoparathyroidism with PTH 1-34. Bone 120:535–541. https://doi.org/10.1016/j.bone.2018.09.018
Winer KK, Kelly A, Johns A, Zhang B, Dowdy K, Kim L, Reynolds JC, Albert PS, Cutler GB Jr (2018) Long-term parathyroid hormone 1-34 replacement therapy in children with hypoparathyroidism. J Pediatr 203:391–399 e391. https://doi.org/10.1016/j.jpeds.2018.08.010
Vahle JL, Long GG, Sandusky G, Westmore M, Ma YL, Sato M (2004) Bone neoplasms in F344 rats given teriparatide [rhPTH(1-34)] are dependent on duration of treatment and dose. Toxicol Pathol 32(4):426–438. https://doi.org/10.1080/01926230490462138
Vahle JL, Sato M, Long GG, Young JK, Francis PC, Engelhardt JA, Westmore MS, Linda Y, Nold JB (2002) Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1-34) for 2 years and relevance to human safety. Toxicol Pathol 30(3):312–321
Catalano A, Vita GL, Russo M, Vita G, Lasco A, Morabito N, Messina S (2016) Effects of teriparatide on bone mineral density and quality of life in Duchenne muscular dystrophy related osteoporosis: a case report. Osteoporosis Int 27(12):3655–3659. https://doi.org/10.1007/s00198-016-3761-x
Gordon CM, Leonard MB, Zemel BS, International Society for Clinical D (2014) 2013 Pediatric Position Development Conference: executive summary and reflections. J Clin Densitom 17(2):219–224. https://doi.org/10.1016/j.jocd.2014.01.007
Braat E, Hoste L, De Waele L, Gheysens O, Vermeersch P, Goffin K, Pottel H, Goemans N, Levtchenko E (2015) Renal function in children and adolescents with Duchenne muscular dystrophy. Neuromuscul Disord 25(5):381–387. https://doi.org/10.1016/j.nmd.2015.01.005
Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM (2003) The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med 349(13):1216–1226. https://doi.org/10.1056/NEJMoa035725
Armbrecht G, Blenk T, Chesnut CH 3rd, Gardner JC, von Ingersleben G, Mahoney P, Felsenberg D (2008) Vertebral fracture diagnosis in the multinational BONE study of oral ibandronate: quality management in radiology. J Clin Densitom 11(2):221–231. https://doi.org/10.1016/j.jocd.2007.10.002
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8(9):1137–1148. https://doi.org/10.1002/jbmr.5650080915
Henderson RC, Lark RK, Newman JE, Kecskemthy H, Fung EB, Renner JB, Harcke HT (2002) Pediatric reference data for dual X-ray absorptiometric measures of normal bone density in the distal femur. AJR Am J Roentgenol 178(2):439–443. https://doi.org/10.2214/ajr.178.2.1780439
Carter DR, Bouxsein ML, Marcus R (1992) New approaches for interpreting projected bone densitometry data. J Bone Miner Res 7(2):137–145. https://doi.org/10.1002/jbmr.5650070204
Kindler JM, Lappe JM, Gilsanz V, Oberfield S, Shepherd JA, Kelly A, Winer KK, Kalkwarf HJ, Zemel BS (2019) Lumbar spine bone mineral apparent density in children: results from the bone mineral density in childhood study. J Clin Endocrinol Metab 104(4):1283–1292. https://doi.org/10.1210/jc.2018-01693
Zemel BS, Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, Frederick MM, Huang X, Lu M, Mahboubi S, Hangartner T, Winer KK (2011) Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab 96(10):3160–3169. https://doi.org/10.1210/jc.2011-1111
Zemel BS, Stallings VA, Leonard MB, Paulhamus DR, Kecskemethy HH, Harcke HT, Henderson RC (2009) Revised pediatric reference data for the lateral distal femur measured by Hologic Discovery/Delphi dual-energy X-ray absorptiometry. J Clin Densitom 12(2):207–218. https://doi.org/10.1016/j.jocd.2009.01.005
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174
Gluer CC, Marin F, Ringe JD, Hawkins F, Moricke R, Papaioannu N, Farahmand P, Minisola S, Martinez G, Nolla JM, Niedhart C, Guanabens N, Nuti R, Martin-Mola E, Thomasius F, Kapetanos G, Pena J, Graeff C, Petto H, Sanz B, Reisinger A, Zysset PK (2013) Comparative effects of teriparatide and risedronate in glucocorticoid-induced osteoporosis in men: 18-month results of the EuroGIOPs trial. J Bone Miner Res 28(6):1355–1368. https://doi.org/10.1002/jbmr.1870
Ettinger B, San Martin J, Crans G, Pavo I (2004) Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res 19(5):745–751. https://doi.org/10.1359/JBMR.040117
Finkelstein JS, Leder BZ, Burnett SM, Wyland JJ, Lee H, de la Paz AV, Gibson K, Neer RM (2006) Effects of teriparatide, alendronate, or both on bone turnover in osteoporotic men. J Clin Endocrinol Metab 91(8):2882–2887. https://doi.org/10.1210/jc.2006-0190
Blumsohn A, Marin F, Nickelsen T, Brixen K, Sigurdsson G, Gonzalez de la Vera J, Boonen S, Liu-Leage S, Barker C, Eastell R, Group ES (2011) Early changes in biochemical markers of bone turnover and their relationship with bone mineral density changes after 24 months of treatment with teriparatide. Osteoporosis Int 22(6):1935–1946. https://doi.org/10.1007/s00198-010-1379-y
Keel C, Kraenzlin ME, Kraenzlin CA, Muller B, Meier C (2010) Impact of bisphosphonate wash-out prior to teriparatide therapy in clinical practice. J Bone Miner Metab 28(1):68–76. https://doi.org/10.1007/s00774-009-0101-7
Stepan JJ, Burr DB, Li J, Ma YL, Petto H, Sipos A, Dobnig H, Fahrleitner-Pammer A, Michalska D, Pavo I (2010) Histomorphometric changes by teriparatide in alendronate-pretreated women with osteoporosis. Osteoporosis Int 21(12):2027–2036. https://doi.org/10.1007/s00198-009-1168-7
Gamsjaeger S, Buchinger B, Zoehrer R, Phipps R, Klaushofer K, Paschalis EP (2011) Effects of one year daily teriparatide treatment on trabecular bone material properties in postmenopausal osteoporotic women previously treated with alendronate or risedronate. Bone 49(6):1160–1165. https://doi.org/10.1016/j.bone.2011.08.015
Fahrleitner-Pammer A, Burr D, Dobnig H, Stepan JJ, Petto H, Li J, Krege JH, Pavo I (2016) Improvement of cancellous bone microstructure in patients on teriparatide following alendronate pretreatment. Bone 89:16–24. https://doi.org/10.1016/j.bone.2016.05.004
Lou S, Lv H, Li Z, Zhang L, Tang P (2018) Combination therapy of anabolic agents and bisphosphonates on bone mineral density in patients with osteoporosis: a meta-analysis of randomised controlled trials. BMJ Open 8(3):e015187. https://doi.org/10.1136/bmjopen-2016-015187
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH (2001) Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344(19):1434–1441. https://doi.org/10.1056/NEJM200105103441904
Tsai JN, Lee H, David NL, Eastell R, Leder BZ (2019) Combination denosumab and high dose teriparatide for postmenopausal osteoporosis (DATA-HD): a randomised, controlled phase 4 trial. Lancet Diabetes Endocrinol 7(10):767–775. https://doi.org/10.1016/S2213-8587(19)30255-4
Miller PD, Bilezikian JP, Diaz-Curiel M, Chen P, Marin F, Krege JH, Wong M, Marcus R (2007) Occurrence of hypercalciuria in patients with osteoporosis treated with teriparatide. J Clin Endocrinol Metab 92(9):3535–3541. https://doi.org/10.1210/jc.2006-2439
Tashjian AH Jr, Goltzman D (2008) On the interpretation of rat carcinogenicity studies for human PTH(1-34) and human PTH(1-84). J Bone Miner Res 23(6):803–811. https://doi.org/10.1359/jbmr.080208
Andrews EB, Gilsenan AW, Midkiff K, Sherrill B, Wu Y, Mann BH, Masica D (2012) The US postmarketing surveillance study of adult osteosarcoma and teriparatide: study design and findings from the first 7 years. J Bone Miner Res 27(12):2429–2437. https://doi.org/10.1002/jbmr.1768
Gilsenan A, Harding A, Kellier-Steele N, Harris D, Midkiff K, Andrews E (2018) The Forteo Patient Registry linkage to multiple state cancer registries: study design and results from the first 8 years. Osteoporosis Int 29(10):2335–2343. https://doi.org/10.1007/s00198-018-4604-8
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethics approval
The study was approved by the Cincinnati Children’s Hospital Institutional Review Board.
Consent to participate
Informed consent and assent were obtained at study enrollment.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nasomyont, N., Keefe, C., Tian, C. et al. Safety and efficacy of teriparatide treatment for severe osteoporosis in patients with Duchenne muscular dystrophy. Osteoporos Int 31, 2449–2459 (2020). https://doi.org/10.1007/s00198-020-05549-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-020-05549-z