Abstract
Summary
Two methods of skeletal status assessment—quantitative ultrasound (QUS) and densitometry (DXA)—were applied and compared in a group of children with different renal disorders. Skeletal assessments in children with different renal conditions should rather not be based on a single diagnostic tool. Lumbar spine DXA is very effective to reveal disturbances secondary to glucocorticoids, whereas total body DXA and QUS are both better in identification of disturbances related to decreased GFR.
Introduction
The aim of the study was to evaluate the skeletal status in children in different stages of chronic kidney disease (CKD) or treated with glucocorticoids, using either densitometry (DXA) or quantitative ultrasound (QUS) methods.
Methods
Seventy-six subjects (27 girls/49 boys) at the mean age of 11.8 ± 4.0 years were enrolled to the reported study. They were divided into three subgroups: with normal glomerular filtration rate (GFR) but treated with glucocorticoids (GCs, n = 38), with decreased GFR (CKD 2–5, n = 26) and with normal GFR and without any bone-toxic treatment (CKD 1, n = 12). DXA scans were carried out at lumbar spine (LS) and at total body (TB), and quantitative ultrasound (QUS) imaging was done at hand phalanges. QUS results were compared to those obtained from 310 healthy matched controls.
Results
The average Z-score for LS-BMD and TB-BMD was below zero in all the study subgroups. Neither were there any significant differences in the mean Z-score for LS among the subgroups. The mean Z-score for TB was significantly the lowest in the CKD 2–5 subgroup. The percentage of subjects with TB Z-score ≤ − 2.0 was the highest in the CKD 2–5 subgroup (69.2%), whereas the percentage of subjects with LS Z-score ≤ − 2.0 was the highest in the GC subgroup (23.7%). QUS results in CKD 2–5 were significantly lower than those in the controls, whereas the results, obtained in GC and CKD 1 subgroups, were similar to those in healthy subjects.
Conclusions
Skeletal status assessment in children and adolescents with different renal conditions should not be based on single diagnostic approach. DXA scanning, performed at lumbar spine, is potentially more appropriate to reveal disturbances secondary to long-term GC therapy, whereas TB-DXA is highly effective in the identification of skeletal disturbances related to decreased kidney function. QUS at hand phalanges seems to be a useful diagnostic means in CKD with diminished GFR but insufficient to detect GC-related disturbances.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal diseases are identified as significant sources of negative impacts on bone metabolism and mineralization [1,2,3,4]. Their effects may result directly from renal dysfunction, e.g., abnormal calcium-phosphorus homeostasis control in tubular transport defects or impaired glomerular filtration rate (GFR) [5,6,7,8]. On the other hand, bone defects, which are found throughout the course of kidney diseases, can be impacted by applied therapy even stronger than by the primary disease. An example of such a situation may be a prolonged use of glucocorticoids (GCs) due to the idiopathic nephrotic syndrome or glomerulonephritis [9,10,11,12].
With regard to adults, both impaired GFR and steroid therapy belong to well-known causes of secondary osteoporosis and are recognized as risk factors for pathological fractures [13].
The knowledge of the association between renal disease and the risk of pathological fractures during the developmental period is neither obvious nor well documented, mainly for difficulties with powerful research projects in big enough cohorts. The approved diagnostic tools, such as bone densitometry (dual-energy X-ray absorptiometry—DXA), are not appropriately validated for direct fracture risk assessments in children and adolescents with CKD [14, 15]. However, regarding pathophysiological prerequisites, it seems appropriate to test the available diagnostic methods in children and adolescents with renal diseases in order to identify discrepancies between observed and expected development trends. Such approach may also help indirectly identify subjects at risk of sub-optimal peak bone mass in early adulthood.
Regardless of some limitations, DXA is the most common diagnostic method to reveal bone defects not only in adults but also during developmental period. There are many examples of clinical DXA applications in pediatric cohorts to reveal disturbances of bone metabolism, e.g., in girls with anorexia nervosa [16], children with end-stage renal disease [6,7,8], or patients with inflammatory bowel disease [17]. The clinical values of DXA measurements are somewhat limited by their very nature, namely that the results (bone mineral density—BMD) are estimated from a single trait—calcium content in bone tissue, while not reflecting possible architectural changes in bone structure (qualitative characteristics) [15]. The advantage of DXA is, however, its possibility to target various skeletal areas with known anatomical and physiological distinctiveness (cortical bone vs. trabecular bone), which may help draw indirect conclusions about the nature of observed disturbances in different pathologies. On the contrary, quantitative ultrasound (QUS) is a method with an expected potential to evaluate both quantitative and qualitative aspects of bone tissue structure [18, 19]. Due to its totally non-invasive character and very short time of single imaging, this method seems to be a reasonable option to evaluate bone changes in different clinical conditions. It is highly interesting if the QUS method could be applied for screening purposes and information, complementary to DXA results.
The aim of the reported study was to evaluate the skeletal status in children and adolescents with different stages of chronic kidney disease (CKD) and under GC therapy, using DXA and QUS diagnostic options to later compare the results of either method.
Material
A group of 76 children and adolescents (27 girls and 49 boys), at the mean age of 11.8 ± 4.0 years, and with chronic kidney disease (CKD) were enrolled into the study. The study subjects were recruited from a single regional center, providing medical care to children with kidney diseases (hospital ward and outpatient clinic).
According to current ‘renal condition’, the study group was divided into the three following subgroups:
-
Children with normal glomerular filtration rate (GFR) but exposed to at least 6-month glucocorticoid therapy, due to the idiopathic nephrotic syndrome (the GC subgroup, n = 38, 12 girls and 26 boys),
-
Children with impaired GFR at consecutive stages of chronic kidney disease (CKD) 2 to 5 but not exposed, either currently or previously to glucocorticoid therapy or to any other treatment with obvious toxicity for bone metabolism (the CKD 2–5 subgroup, n = 26, 10 girls and 16 boys). The numbers of children at consecutive CKD stages: n = 3 with CKD 2, n = 7 with CKD 3, n = 4 with CKD 4, and n = 12 with CKD 5,
-
Children with both CKD and preserved normal GFR, not exposed, either currently or previously to glucocorticoid therapy or to any other treatment with obvious toxicity for bone metabolism—e.g., mild proteinuria or early stages of chronic tubulo-interstitial nephritis (the CKD 1 subgroup, n = 12, 5 girls and 7 boys).
In order to avoid any bias related to unclear qualification to analyzed subgroups, the subjects with mixed risk factors (e.g., reduced GFR together with exposure to glucocorticoids) were not taken into consideration.
Clinical characteristics of the studied subjects are presented in Table 1.
All the identified disturbances, either potentially reversible or correctable, which could additionally affect the bone status (e.g., hypothyroidism in nephrotic children, secondary hyperparathyroidism, previously detected vitamin D deficiency, metabolic acidosis, etc) were treated according to clinical standards.
For comparative analyses related to QUS measurements, a control group was arranged with 310 age-, sex-, and body size-matched healthy children, selected from a previously published study [20]. The quantitative proportion of controls to study subjects was 5 to 1. As the study subgroups differed with regards to their age and body size, the controls were separately selected for GC, CKD 2–5, and CKD 1 to achieve an optimal matching between the patients and their healthy counterparts. The number of healthy children in each of the such selected control subgroups was proportional to the number of study subjects in each of the study subgroups, thus guaranteeing that proper age-, sex-, and body size-matching had been maintained between the whole study group and the control group.
The study design was approved by the Ethics Committee of the Medical University of Silesia (Katowice, Poland). The patients’ parents or caregivers gave their informed, written consent to enrolment of their children and/or dependants into the study.
Methods
Bone measurements
The skeletal status of study participants was assessed by QUS and DXA measurements.
QUS measurements were performed at hand phalanges with a DBM Sonic 1200 (IGEA, Carpi, Italy) device. The DBM Sonic device consists of two probes, mounted on an electronic caliper: one being the emitter and the other the receiver of ultrasound signals. The acoustic coupling was achieved, using a standard ultrasound gel. Amplitude-dependent sound speed (Ad-SoS [m/s]) was measured at distal metaphyses of the proximal phalanges of fingers II–V of the dominant hand, taking into account the first signal with amplitude of, at least, 2 mV at the receiving probe. Thus, the measured speed of sound was specified as ‘amplitude dependent’. The mean value of the results, obtained from 4 fingers, was then calculated and used in further analyses.
In order to achieve normalization of Ad-SoS results for age and gender, Z-score values were also calculated. Z-score depicts the number of SDs (standard deviation—SD) from the mean Ad-SoS values, calculated for age- and sex-matched cohort, derived from reference population. Individual Z-scores are calculated, according to the following formula:
The results from a previously performed population-based study were used as normative values [20].
An in vivo precision of measurements was previously established on the basis of five repeated measurements and expressed as root-mean-square coefficient of variation (CV%). Its value was 0.64% [20]. The sonographic device was calibrated before each measurement session, according to the manufacturer’s recommendations. All the measurements were performed by the same experienced operator (W.P.).
Bone densitometry was performed, using a Hologic Explorer (Hologic Inc., Waltham, MA, USA; software version: 13.0.3). BMD (g/cm2) was measured at lumbar spine level (LS-BMD) and for total body (TB-BMD). The scans of total body were analyzed with the ‘auto whole body’ option incorporated in the software of the densitometer. The obtained results were also transformed as Z-scores, using pediatric gender-specific and age-matched reference data provided by the manufacturer of the densitometer. All the examinations were performed by one experienced technician. The precision of measurements for LS-BMD was established on the basis of 50 measurements (two scans in 25 subjects) and expressed as CV% equal 1.6%. CV% for TB-BMD, according to data given by the manufacturer, was 1.8%.
Statistics
A statistical analysis was performed, using the Statistica software (StatSoft, Tulsa, OK, USA). Descriptive statistics for continuous variables were presented as mean values and standard deviations. The normality of data distribution was verified by the Shapiro-Wilk test. The Student’s t test or the Mann-Whitney U test (for data with and without normal distribution, respectively) was employed for comparative analyses between studied subjects and controls, as well as between subgroups derived from the study group. Qualitative variables were presented as percentage values and compared between groups with the Chi-square test. Correlation analysis was done by Pearson’s or Spearman’s correlation tests, whichever was appropriate, according to data character (continuous or categorized) and normality of distribution. A multivariate analysis was performed, regarding the identification of independent factors, influencing QUS and DXA results, with the application of multiple stepwise regression. Significance for obtained results of all the statistical analyses was assumed at p < 0.05.
Results
DXA results in subgroups
DXA results, obtained both at lumbar spine and in total body scans and presented as BMD values and Z-scores, as well as total body bone mineral content (TB BMC) values are provided in Table 2. As the analyzed subgroups differed in the mean age of subjects, the Z-score values were considered for further interpretation. There were no significant differences in the mean Z-scores for LS among the subgroups. The mean Z-score for TB was the lowest in CKD 2–5 subgroup and differed significantly from the TB Z-score in GC patients.
The mean Z-score values below zero (i.e., lower than the mean values for healthy counterparts), observed in all the subgroups and for both DXA modalities, suggest the presence of bone mineralization disturbances in the studied subjects but even more informative is the interpretation of individual results. Table 3 shows the percentage of children with Z-score above − 1.00, between − 1.00 and − 1.99 and equal or below − 2.00 in the derived subgroups. It can be noticed that the strongest disturbances were revealed in CKD 2–5 patients with TB scan—almost 70% of patients had their Z-score below − 2.0 (significantly higher rate than in other subgroups; Chi-square test; p < 0.05). What is of interest, the LS scanning provided much more ‘optimistic’ results in that subgroup—only 12.5% of CKD 2–5 children had their LS Z-score below − 2.0. Such a deep discrepancy between DXA modalities was not noted in GC children (23.7 and 39.5% with Z-score below − 2.0, according to LS and TB scan, respectively). As expected, the lowest rate of children with Z-score below − 2.0 for both DXA methods was noted in the subgroup without exposure to steroids and with normal GFR (CKD 1).
QUS results in the studied subjects and their comparison to the results in the controls.
The results of QUS measurements (Ad-SoS [m/s] and Z-scores), obtained in the subgroups of the study group and in healthy sex-, age-, and body size-matched counterparts, are presented in Table 4. The QUS results were significantly lower in children with impaired renal function than in healthy children, whereas the results in children, treated with steroids and those from the CKD 1 group, did not differ significantly from the results in the controls. Moreover, the mean Z-score for Ad-SoS in the CKD 2–5 subgroup was significantly lower than in the GC subgroup. The percentage of subjects with Z-score for Ad-SoS below − 2.0 was the highest in the CKD 2–5 subgroup, reaching 40%, thus revealing significant differences in comparison to 6.5% in the GC subgroup and 16.7% in the CKD 1 subgroup (Chi-square test; p < 0.05). It can be summarized that the QUS method, applied for imaging at hand phalanges, revealed bone disturbances only in children with decreased GFR.
Correlation between skeletal and clinical parameters
As the parameters, measured directly by DXA (BMD) and QUS (Ad-SoS), are strongly determined by age and body size during developmental period in both physiological and pathological conditions, correlation analyses were performed with Z-score values.
In the GC subgroup, Z-score for LS correlated negatively with the age (r = − 0.44; p < 0.01), disease duration (r = − 0.50; p < 0.01), the time period of steroid therapy (r = − 0.52; p < 0.001), and positively with SDS for body weight (r = 0.47; p < 0.01) and SDS for body height (r = 0.65; p < 0.001). Very similar dependencies were observed with Z-scores for TB: a negative correlation with the age (r = − 0.48; p < 0.01), disease duration (r = − 0.56; p < 0.001), the time period of steroid therapy (r = − 0.51; p < 0.001), and a positive correlation with SDS for body weight (r = 0.54; p < 0.001) and SDS for height (r = 0.67; p < 0.001). On the contrary, the Z-score for Ad-SoS revealed only a single significant correlation with SDS for body height (r = 0.45; p < 0.01).
Significant correlations were found in the CKD 2–5 subgroup between skeletal and anthropometric parameters: the Z-score for LS correlated positively with SDS for body weight (r = 0.50; p < 0.05) and SDS for body height (r = 0.41; p < 0.05), the Z-score for TB–with SDS for body weight (r = 0.75; p < 0.001), and SDS for body height (r = 0.67; p < 0.001) and the Z-score for Ad-SoS–with SDS for body height (r = 0.51; p < 0.01). The influence of age, the age at onset of disease, and disease duration on skeletal parameters remained insignificant in that subgroup. However, when the CKD 2–5 subgroup was combined with the CKD 1 subgroup (i.e., patients at 1 to 5 stages of CKD, except of those on glucocorticoids), a significant negative rank correlation was found between the stage of the disease and the Z-score for TB (r = − 0.44; p < 0.01), as well as between the stage of the disease and the Z-score for Ad-SoS (r = − 0.42; p < 0.05).
In the CKD 1 subgroup, the only significant correlations were found with SDS for body weight: with the LS Z-score (r = 0.67; p < 0.05) and with the TB Z-score (r = 0.59; p < 0.05).
Correlation between the methods applied for skeletal status assessment
Due to essential discrepancies between the results of DXA and QUS methods in all the analyzed subgroups, a correlation was also checked between the applied diagnostic tools. A very strong positive correlation was confirmed among BMD values, measured in both DXA modalities (LS and TB), in the whole study group, as well as in the subgroups with r values for all the children, GCs, CKD 2–5, and CKD 1: 0.88 (p < 0.0001), 0.85 (p < 0.0001), 0.93 (p < 0.0001), and 0.94 (p < 0.0001), respectively. When the Z-scores were analyzed instead of BMD, the respective r values for all the children, GCs, CKD 2–5, and CKD 1 were as follows: 0.71 (p < 0.0001), 0.72 (p < 0.0001), 0.86 (p < 0.0001), and 0.91 (p < 0.0001).
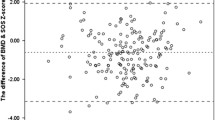
The correlations between QUS and DXA were not so unequivocal. When the directly measured parameters (BMD vs. Ad-SoS) were correlated, the strongest connection was found between Ad-SoS and TB BMD in the CKD 2–5 subgroup (r = 0.77; p < 0.0001). Weaker but still significant correlations were also noticed between Ad-SoS and LS BMD in the CKD 2–5 subgroup, as well as between Ad-SoS and both TB BMD and LS BMD in the other subgroups (r value range 0.42–0.69). However, when the Z-scores were applied instead of Ad-SoS and BMD (which eliminates the influence of the age-dependent variability of measured parameters on correlations among them), only the correlation of the Z-score for Ad-SoS with Z-score for TB in the whole study group (r = 0.45; p < 0.001) and in the CKD 2–5 subgroup (r = 0.40; p < 0.05) remained significant. Figure 1 presents divergent correlations among Z-scores for Ad-SoS versus Z-scores for TB-BMD and Z-scores for LS-BMD in the whole study group.
Multiple stepwise regression analysis
A multiple stepwise regression analysis was performed in order to identify the significance of the influence of physiological factors and some risk factors related to kidney disease in multivariate analysis. Ad-SoS, LS BMD, and TB BMD were regressed on age, sex, Tanner stage, body mass, body height (physiological factors), as well as on the age at the onset of the disease, duration of the disease, exposure to glucocorticoids, and the stage of CKD (conditions related to the disease). The results of multiple stepwise regression analyses are presented as regression equations in Table 5.
It is worth noting that the multiple stepwise regression analysis allowed to reveal that the exposure to glucocorticoids had a negative influence on LS BMD value but not on Ad-SoS or TB BMD. On the contrary, the progression of CKD, expressed as CKD stage, influenced negatively Ad-SoS and TB BMD but not LS BMD. The influence of age and physical development parameters was generally concordant with the trends observed in the healthy population. Neither the age at the onset of the disease nor duration of the disease was identified as an independent significant factor in the performed analyses.
Discussion
It is not questionable that mineral and bone disturbances, even if clinically silent or often oligosymptomatic for many years, constitute an important clinical problem in patients with chronic kidney disease. Their impact on the general health status and the quality of life is potentially more pronounced in case of kidney diseases with early age onset. First, by expected major implications of disorders, beginning in the developmental period (e.g., significantly reduced peak bone mass), and second, due to the relatively long expected survival period of subjects affected by this problem [3]. Both aspects result in significantly higher bone fragility throughout their life.
The key issue in practical management is then the availability of diagnostic tools to accurately identify patients at risk of bone complications, as well as to assess the severity of complications and determine their specificity—adequately enough for available therapeutic interventions. One of the possible tools, characterized by wide availability, relative simplicity, and low cost, used for prognostic assessments of fracture risk, is DXA. DXA has been validated in various groups of patients. Unfortunately, the available data suggest some difficulties in the interpretation of DXA results during developmental period [14], as well as in bone abnormalities, secondary to renal disease [15]. However, regardless of the inability to quantify the risk of fracture, this method demonstrates distinct differences in bone development patterns between young patients with renal diseases and their healthy counterparts.
Being aware of the limitations of separate DXA measurements at single skeletal site, the authors of this study attempted skeleton assessments in children and adolescents with kidney diseases in two different projections of DXA (LS and TB), together with QUS imaging at hand phalanges. The obtained results showed that the simultaneous use of those three diagnostic options significantly increased the sensitivity of detection of bone disorders. Regarding DXA at lumbar spine, it revealed GC-dependent abnormalities of bone mineralization, while the TB projection seemed to be more effective in identifying bone disturbances in patients with impaired GFR. The observed in our study and seemingly potential usefulness of TB DXA in the identification of bone disorders in patients with impaired GFR may be explained in such a way that, although this DXA examination modality covers the whole skeleton, its results are predominantly determined by the status of cortical bone mineralization. Bone disorders secondary to progressive glomerular filtration rate deterioration have complex etiology, but their expected key mechanism is related to the development of secondary hyperparathyroidism. Demineralization dependent on parathyroid hormone overproduction affects mainly the cortical bone [21]. This may be the explanation for the wide occurrence of disorders identified by reduced BMD in TB DXA in the CKD 2–5 subgroup, while at the same time, relatively good results of LS DXA are noted.
QUS results showed a strong correlation with the results of TB-BMD and allowed to demonstrate significant differences in patients with abnormal GFR, when compared to healthy subjects. On the contrary—the correlation between QUS and LS-BMD was rather weak, and the use of QUS in the subgroup, treated with GCs, did not allow to detect any differences between the subjects and the controls.
A parallel use of densitometry and ultrasound in various modifications has also been applied in other studies. Christoforidis et al. [22] compared the use of DXA at LS and QUS in radius and tibia imaging in a group of 20 children with CKD, demonstrating the lack of association between the methods. In addition, the authors showed that QUS more strongly correlated with some selected biochemical markers of renal osteodystrophy, suggesting that ‘QUS was more useful than DXA in evaluating bone disease in young patients with CKD’. Nevertheless, the study did not adopt other variants of DXA, in particular, TB measurement. The same group of authors published a prospective study in a group of pediatric kidney transplant recipients [23]. Two DXA modalities (LS and proximal femur), as well as QUS at radius and tibia, were employed to monitor the longitudinal changes during a 2-year period. A clear discrepancy was found among the methods: an improvement in QUS parameters was accompanied by deterioration in DXA measurements. It can be speculated that the observed positive trend in QUS results reflected the resolution of CKD-related skeletal changes, achieved due to normalization of GFR after renal transplantation, whereas the deterioration in DXA results should be considered as an adverse effect of GC-based immunosuppression. Regardless of the differences in study design, this phenomenon of bifurcated trends of QUS and DXA evolution may be perceived as similar to the incoherent findings in our group with DXA at LS, sufficient to detect bone disturbances in GC-treated patients and QUS or TB-DXA, revealing changes related to kidney function deterioration. Similar differences were found by Gkogka et al. between longitudinal trends for radius or tibia QUS and DXA results for LS or proximal femur in a study, performed in pediatric patients on continued dialysis treatment or after kidney transplantation [5].
Gonzalez-Jorge et al. conducted a study, based on DXA measurements at LS and TB and QUS at radius in a group of children, assessed shortly after kidney transplantation [24]. The highest percentage of patients with a significant degree of impaired bone mineralization was identified with TB-BMD. QUS correlated with both variants of densitometry; however, in individual assessments, there were many patients with osteoporosis/osteopenia according to DXA who were identified by QUS as subjects with normal bone mineralization.
The divergence between densitometric and ultrasonic assessment of skeletal disorders, observed in our study, as well as reported by many other authors, has at least two potentially important sources. First, bone disorders, which are secondary to kidney diseases, although generalized in assumption, may achieve different intensity in particular skeletal sites. Second, the diagnostic tools, employed in our study and used by the authors of cited works, do not allow for a fully separate assessment of cortical and trabecular bones, and these two types of bone tissue are usually affected to a different extent in various pathological conditions. The latter statement is supported by the results of the studies with separate assessment of cortical and trabecular bone, based on the method of high-resolution peripheral quantitative computed tomography (HR-pQCT) [25, 26]. DXA and QUS measurements, performed at different skeletal sites, are, to some extent, more dependent on the status of either cortical or trabecular bone, but they should never be regarded as specific to one type of bone tissue only. Based on theoretical premises and studies, comparative to the HR-pQCT method [26], it can be assumed that LS-BMD is more dependent on the state of trabecular bone, whereas TB-BMD reflects better the state of cortical bone. QUS measurements at hand phalanges are also more representative for cortical bone. Further studies, based on QUS measurements at other skeletal sites, e.g., calcaneal or tibial QUS, applied together with different DXA modalities and, optimally with HR-pQCT, carried out in children with CKD of different etiology (and in other groups of patients as well), could provide some new, interesting data verifying our findings derived from the current study.
The presented study had some limitations. The study group was not numerous, especially after dividing into subgroups, although, taking into account the epidemiology of CKD in children, it can be stated that the cohort was not small, either. The QUS measurements were performed at one skeletal site only. No biochemical markers were available, either of vitamin D status or bone turnover. Comparisons to healthy controls were possible for the QUS measurements only, as DXA was not performed in healthy volunteers, due to ethical reasons. DXA measurements for total body were analyzed with the ‘auto whole body’ option, provided in the software of the used densitometer, whereas the ‘total body less head’ analysis is currently recommended as a preferable option for clinical purposes [14]. The pubertal status in the controls was not described. One should be aware that the clinical usefulness of obtained and presented results is currently limited pending the expected improvement in accuracy of diagnostic tools evaluating the extent, rate, and dominant location of disorders in bone mineralization and structure.
In conclusion, skeletal status assessments in children and adolescents with different renal conditions should not be based on single diagnostic technique. According to our experience, DXA at lumbar spine is most appropriate to reveal bone mineralization disturbances secondary to long-term GC therapy. Total body DXA is more sensitive to detect skeletal disturbances related to decreased kidney function. QUS at hand phalanges, being a simple and fairly inexpensive method, may be considered for screening purposes in CKD with diminished glomerular filtration rate, whereas it is totally insufficient to detect GC-related disturbances. However, the wide application of QUS in clinical practice requires a thorough validation of that method. An interpretation of any skeletal parameters, based on single diagnostic tool, should be always provided with caution, as it can be not sensitive enough to recognize subjects at higher risk of bone problems. Further studies, based on more advanced diagnostic techniques, such as HR-pQCT, are postulated to verify the utility of either complementary or alternative applications of different QUS and DXA modalities in various clinical conditions.
References
Kidney Disease Outcomes Quality Initiative (K/DOQI) (2005) Clinical practice guidelines for bone metabolism and disease in children with chronic kidney disease. Am J Kidney Dis 46(Suppl 1):S1–S103
Moe S, Drüeke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G, Kidney Disease: Improving Global Outcomes (KDIGO) (2006) Definition, evaluation, and classification of renal osteodystrophy: a position statement from kidney disease: improving global outcomes (KDIGO). Kidney Int 69:1945–1953
Leonard MD (2007) A structural approach to the assessment of fracture risk in children and adolescents with chronic kidney disease. Pediatr Nephrol 22:1815–1824
Sanchez CP (2008) Mineral metabolism and bone abnormalities in children with chronic renal failure. Rev Endocr Metab Disord 9:131–137
Gkogka C, Christoforidis A, Printza N, Kollios K, Kazantzidou E, Papachristou F (2015) Longitudinal assessment of bone quality in pediatric patients with chronic kidney disease in relation to treatment modality. J Bone Miner Metab 33:303–310
Pluskiewicz W, Adamczyk P, Drozdzowska B, Szprynger K, Szczepanska M, Halaba Z, Karasek D (2002) Skeletal status in children, adolescents and young adults with end-stage renal failure treated with hemo- or peritoneal dialysis. Osteoporos Int 13:353–357
Pluskiewicz W, Adamczyk P, Drozdzowska B, Szprynger K, Szczepańska M, Halaba Z, Karasek D (2003) Skeletal status in children and adolescents with chronic renal failure before onset of dialysis or on dialysis. Osteoporos Int 14:283–288
Pluskiewicz W, Adamczyk P, Drozdzowska B, Szprynger K, Szczepańska M, Halaba Z, Karasek D (2005) Skeletal status in adolescents with end-stage renal failure: a longitudinal study. Osteoporos Int 16:289–295
Hegarty J, Mughal MZ, Adams J, Webb NJ (2005) Reduced bone mineral density in adults treated with high-dose corticosteroids for childhood nephrotic syndrome. Kidney Int 68:2304–2309
Wetzsteon RJ, Shults J, Zemel BS, Gupta PU, Burnham JM, Herskovitz RM, Howard KM, Leonard MB (2009) Divergent effects of glucocorticoids on cortical and trabecular compartment BMD in childhood nephrotic syndrome. J Bone Miner Res 24:503–513
Tsampalieros A, Gupta P, Denburg MR, Shults J, Zemel BS, Mostoufi-Moab S, Wetzsteon RJ, Herskovitz RM, Whitehead KM, Leonard MB (2013) Glucocorticoid effects on changes in bone mineral density and cortical structure in childhood nephrotic syndrome. J Bone Miner Res 28:480–488
Aceto G, D'Addato O, Messina G, Carbone V, Cavallo L, Brunetti G, Faienza MF (2014) Bone health in children and adolescents with steroid-sensitive nephrotic syndrome assessed by DXA and QUS. Pediatr Nephrol 29:2147–2155
Kanis JA, on behalf of the World Health Organization Scientific Group (2007) Assessment of osteoporosis at the primary health-care level. Technical Report. World Health Organization Collaborating Centre for Metabolic Bone Diseases, University of Sheffield
Bianchi ML1, Baim S, Bishop NJ, Gordon CM, Hans DB, Langman CB, Leonard MB, Kalkwarf HJ (2010) Official positions of the International Society for Clinical Densitometry (ISCD) on DXA evaluation in children and adolescents. Pediatr Nephrol 25:37–47
Weber LT, Mehls O (2010) Limitations of dual x-ray absorptiometry in children with chronic kidney disease. Pediatr Nephrol 25:3–5
Oświecimska J, Ziora K, Pluskiewicz W, Geisler G, Broll-Waśka K, Karasek D, Dyduch A (2007) Skeletal status and laboratory investigations in adolescent girls with anorexia nervosa. Bone 41:103–110
Bąk-Drabik K, Adamczyk P, Chobot A, Kwiecień J, Pluskiewicz W (2016) Bone status assessed by quantitative ultrasound in children with inflammatory bowel disease: a comparison with DXA. Expert Rev Gastroenterol Hepatol 10:1305–1312
Nicholson PH, Müller R, Cheng XG, Rüegsegger P, Van Der Perre G, Dequeker J, Boonen S (2001) Quantitative ultrasound and trabecular architecture in the human calcaneus. J Bone Miner Res 16:1886–1892
Njeh CF, Fuerst T, Diessel E, Genant HK (2001) Is quantitative ultrasound dependent on bone structure? A reflection. Osteoporos Int 12:1–15
Halaba ZP, Pluskiewicz W (2004) Quantitative ultrasound in the assessment of skeletal status in children and adolescents. Ultrasound Med Biol 30:239–243
Nickolas TL, Leonard MB, Shane E (2008) Chronic kidney disease and bone fracture: a growing concern. Kidney Int 74:721–731
Christoforidis A, Printza N, Gkogka C, Siomou E, Challa A, Kazantzidou E, Kollios K, Papachristou F (2011) Comparative study of quantitative ultrasonography and dual-energy X-ray absorptiometry for evaluating renal osteodystrophy in children with chronic kidney disease. J Bone Miner Metab 29:321–327
Christoforidis A, Printza N, Gkogka C, Kazantzidou E, Papachristou F (2013) A two-yr prospective study of bone health in children after renal transplantation employing two imaging techniques. Clin Transpl 27:710–717
González-Jorge AL, Enciso S, Reyes A, Hernández AM, Ortiz L, Aldana R, Velásquez-Forero F, Ambrosi R, Clark P, Méndez L, Rivas-Ruiz R, Medeiros M (2016) Post-renal transplantation bone health in children evaluated by means of quantitative ultrasound and densitometry. Transplant Proc 48:635–638
Bacchetta J, Boutroy S, Vilayphiou N, Ranchin B, Fouque-Aubert A, Basmaison O, Cochat P (2011) Bone assessment in children with chronic kidney disease: data from two new bone imaging techniques in a single-center pilot study. Pediatr Nephrol 26:587–595
Griffin LM, Kalkwarf HJ, Zemel BS, Shults J, Wetzsteon RJ, Strife CF, Leonard MB (2012) Assessment of dual-energy X-ray absorptiometry measures of bone health in pediatric chronic kidneydisease. Pediatr Nephrol 27:1139–1148
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study design was approved by the Ethics Committee of the Medical University of Silesia (Katowice, Poland). The patients’ parents or caregivers gave their informed, written consent to enrolment of their children and/or dependants into the study.
Conflicts of interest
None.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Adamczyk, P., Szczepanska, M. & Pluskiewicz, W. Skeletal status assessment by quantitative ultrasound and bone densitometry in children with different renal conditions. Osteoporos Int 29, 2667–2675 (2018). https://doi.org/10.1007/s00198-018-4659-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-018-4659-6