Abstract
Summary
In this post hoc analysis of the VITdAL-ICU study, an RCT in critically ill adults with 25-hydroxyvitamin D levels ≤20 ng/ml, vitamin D3 did not have a significant effect on β-Crosslaps and osteocalcin.
Introduction
Observational studies have shown accelerated bone loss in ICU survivors. A reversible contributor is vitamin D deficiency. In a post hoc analysis of the VITdAL-ICU study, we evaluated the effect of high-dose vitamin D3 on the bone turnover markers (BTM) β-Crosslaps (CTX) and osteocalcin (OC).
Methods
The VITdAL-ICU study was a randomized, double-blind, placebo-controlled trial in critically ill adults with 25-hydroxyvitamin D levels ≤20 ng/ml who received placebo or high-dose vitamin D3 (a loading dose of 540,000 IU and starting 1 month after the loading dose five monthly maintenance doses of 90,000 IU). In this analysis on 289 survivors (209 telephone, 80 personal follow-up visits), BTM were analyzed on days 0, 3, 7, 28, and 180; self-reported falls and fractures were assessed. Bone mineral density (BMD) was measured after 6 months.
Results
At baseline, CTX was elevated; OC was low in both groups—after 6 months, both had returned to normal. There were no differences between groups concerning BTM, BMD, falls, or fractures. In linear mixed effects models, CTX and OC showed a significant change over time (p < 0.001, respectively), but there was no difference between the vitamin D and placebo group (p = 0.688 and p = 0.972, respectively).
Conclusions
Vitamin D supplementation did not have a significant effect on BTM. Further studies should assess the effectiveness of vitamin D on musculoskeletal outcomes in ICU survivors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In addition to underlying disease, critical illness per se seems to be detrimental to musculoskeletal health in various ways [1,2,3]: immobilization, inflammation, multiple endocrine alterations, hypercatabolism including muscle wasting, malnutrition, and certain drugs all have the potential to disturb the delicate balance between bone formation and bone resorption. Immobilization rapidly induces high bone turnover and bone loss in healthy adults and in patients following stroke with consecutive palsy [4, 5]. Inflammation-induced cytokines like TNF-α and IL-6 are elevated in critical illness [6], may reach extreme levels during sepsis, and induce bone resorption through osteoclastogenesis. Furthermore, hormonal and metabolic changes with adverse effects on bone that frequently affect critically ill patients include hypercortisolism, hypogonadism, hyposomatotropism, and secondary hypothyroidism besides alterations in calcium-phosphate metabolism and parathyroid hormone secretion [7, 8]. Finally, glucocorticoids, proton pump inhibitors, loop diuretics, and catecholamines may have negative effects on skeletal integrity [9,10,11,12]. Vitamin D deficiency also has negative consequences on musculoskeletal health [3].
Data on skeletal consequences of critical care including bone turnover, bone density, and fracture risk are limited. Under normal conditions, bone formation and bone resorption are tightly linked. In critical illness, however, marked uncoupling between osteoblast and osteoclast functions seems to be present [3]. A similar constellation is found in transplantation bone disease and glucocorticoid-induced osteoporosis, both conditions in which bone may be lost very rapidly accompanied by high fracture rates [12,13,14]. Bone resorption markers are high and increase further during intensive care unit (ICU) stay [3, 6, 15, 16] especially in sepsis [17] and following burn injuries [18, 19].
In 2011, a retrospective case-cohort study in 258 women and 481 men reported an increased fracture risk following critical illness; however, vitamin D status was not available in this study [20]. The same group recently demonstrated in a prospective observational study that bone loss in 66 patients was significantly greater in the year following ICU compared to controls from the Geelong Osteoporosis study [21].
More than a decade ago, Greet Van den Berghe et al. reported an effect of “high-dose” intravenous cholecalciferol supplementation (daily vitamin D supplement of ± 500 IU vitamin D3) on bone turnover markers in prolonged critical illness [6]. Vitamin D slightly increased serum osteocalcin (OC) and diminished carboxy terminal propeptide type-I collagen; other bone turnover markers were not affected, however [6]. In patients with arterial hypertension and vitamin D deficiency no significant effect of vitamin D supplementation on bone turnover markers could be observed [22]. In older community-dwelling women, annual oral administration of high-dose cholecalciferol even resulted in an increased risk of falls and fractures [23]. Following heart transplantation, a higher rate of bone loss is associated with lower vitamin D serum levels [14]. Most importantly, vitamin D deficiency is one of the few modifiable risk factors for compromised skeletal health during and following critical illness. In addition, in their scientific opinion paper published in 2016, the European Food Safety Authority (EFSA) stated that “more research is needed to establish the relationship between responses of bone markers (e.g., OC, bone ALP, and urine N-telopeptide crosslinks) to changes in vitamin D status” [24]. Especially in light of the contradictory evidence published on vitamin D supplementation and BMD and fracture risk, more data on faster changing parameters than BMD and fracture development are of interest to detect more subtle effects of vitamin D on bone [24].
We therefore aimed to evaluate the effect of vitamin D supplementation on bone turnover markers in critical illness survivors as a primary outcome in a post hoc analysis of the VITdAL-ICU study, our recent phase III study evaluating the effect of high-dose vitamin D3 on hospital length of stay [25]. Further, we aimed to compare bone mineral density (BMD) and self-reported falls and fractures 6 months after an ICU stay in patients having received placebo versus patients having received vitamin D.
Material and methods
Study design
The detailed study design and methods as well as the results regarding the primary and secondary outcomes including hospital length of stay, length of ICU stay, the percentage of patients with 25-hydroxyvitamin D levels higher than 30 ng/ml at day 7, hospital mortality, and 6-month mortality have been reported previously [25, 26]. In brief, the VITdAL-ICU study was conducted at the Medical University of Graz, a large tertiary academic center in the southeast of Austria with 1538 beds including 123 ICU beds at five intensive care units (1 medical, 1 neurological, 3 surgical units). Adult patients who were expected to stay in the ICU ≥48 h were screened for vitamin D deficiency by measurement of serum 25(OH)D (measured with the IDS-iSYS, an assay based on chemiluminescence technology (Immunodiagnostic Systems, Boldon, UK)).
Major exclusion criteria were severely impaired gastrointestinal motility, pregnant or lactating women, hypercalcemia (total calcium >2.65 mmol/l or ionized serum calcium >1.35 mmol/l), tuberculosis, sarcoidosis, or nephrolithiasis within the prior year.
In accordance with national and EU requirements and the principles of the Declaration of Helsinki [26], written informed consent was waived, delayed, or obtained from a legal surrogate, depending on the circumstances, and was obtained from each patient who regained mental capacity. The study had previously been registered at clinicaltrials.gov (Identifier: NCT01130181).
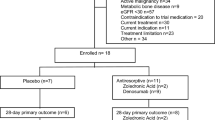
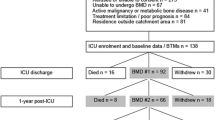
For the present analyses, only patients who had a 6-month-follow-up—either a personal or a telephone visit—were included.
Randomization and intervention
Patients were randomly assigned to either a “placebo” or “vitamin D” group in a 1:1 ratio, using the “Randomizer for Clinical Trials” tool developed at the Medical University of Graz (www.randomizer.at), stratified according to ICU type and gender. Patients randomized to the vitamin D group received a loading dose of 540,000 IU of vitamin D3 and starting 1 month after the loading dose, five monthly maintenance doses of 90,000 IU oral vitamin D3 or respective placebo. About 90% of patients in both groups did actually receive the monthly doses. All trial participants, investigators, and assessors were blinded. Patients of both treatment arms were allowed to receive standard vitamin D supplements via enteral and/or parenteral nutrition (approximately 200 IE per day) at the discretion of the treating physician.
Outcomes and follow-up
We followed all patients for 6 months, assessing among other variables bone turnover markers as well as the number of falls and fractures. BMD measurements were performed at the lumbar spine and femoral neck (Lunar iDXA®, GE) in the self-selected subgroup of patients who came for a follow-up visit after 6 months. Patients were free to choose personal (n = 80) or telephone (n = 210) 6-month visits based on their preference.
Laboratory analysis
Blood and urine samples were collected on days 0, 3, 7, 28, and where feasible, month 6. OC and ß-Crosslaps (CTX) levels were measured in all 289 patients at baseline and after 6 months in the 80 patients who agreed to a personal follow-up visit at our clinic. CTX levels were measured by electrochemiluminescence immunoassay (Cobas, Roche, Mannheim, Germany). Total serum OC was also measured by electrochemiluminescence immunoassay (Cobas, Roche) according to the manufacturer’s instructions.
Data management
Investigators who were blinded to study-group assignments collected data. Only potential study drug-related adverse events (hypercalcemia, hypercalciuria, mortality, falls, fractures) were monitored and recorded within the 6-month follow-up.
Statistical analysis
Continuous data following a normal distribution are shown as means with standard deviations; parameters with a skewed distribution as medians with interquartile ranges and categorical data are presented as percentages. To compare between the vitamin D and placebo group at baseline, the unpaired Student t-test, the Mann-Whitney-U-test, or the chi-squared test were used. To evaluate changes in bone turnover markers over time and to compare changes in bone turnover markers between the vitamin D and the placebo group, a linear mixed effects model was calculated.
Where appropriate, skewed variables were ln transformed for parametric analyses. A two-sided p-value of less than 0.05 was considered significant. Analyses were performed with SAS, version 9.2, and SPSS, version 22.
Funding
The study was supported by the European Society for Clinical Nutrition and Metabolism (ESPEN), a research grant including provision of study medication from Fresenius Kabi (Germany) and the Austrian National Bank (ÖNB Jubiläumsfonds, Project Nr. 14,143). The funding sources had no influence on the design of the study, data analysis, or manuscript preparation.
Results
Two hundred eighty-nine of the patients recruited between May 2010 and March 2012 [25] had a follow-up visit after 6 months. Eighty patients presented personally in the outpatient clinic after 6 months, the other 209 survivors were evaluated by telephone. Reasons for not returning for a follow-up in person were of geographical nature and the patients’ clinical conditions. Baseline demographic and clinical characteristics of the 289 patients according to vitamin D or placebo were comparable in the two groups (Table 1).
In linear mixed effects models, 25(OH)D showed a significant change over time (p < 0.001) and between the vitamin D and placebo group (p < 0.001). 1,25(OH)D showed a significant change over time (p < 0.001), but not between groups (p = 0.148), as did total calcium (over time: p < 0.001; between groups: p = 0.365), ionized calcium (over time: p < 0.001; between groups: p = 0.242), urinary calcium/creatinine-ratio (over time: p < 0.001; between groups: p = 0.409), as well as CTX (over time: p < 0.001; between groups: p = 0.688)—which decreased—or OC (over time: p < 0.001; between groups: p = 0.972)— which increased. Despite the significant increase, OC remained within normal levels. Sclerostin also showed a significant change over time (p = 0.016), but not between groups (p = 0.140).
There was, however, a significant difference over time as well as between groups for phosphate (over time: p = 0.011; between groups: p = 0.030) and parathyroid hormone (over time: p < 0.001; between groups: p = 0.038) Table 2.
Two clinical fractures occurred in each group within 6 months (spine X-rays were not performed at follow-up). Rates of self-reported falls at 6 months were numerically higher in the placebo group (33/136, 24.3%, in the placebo group vs. 27/153, 17.7%, in the vitamin D group, p = 0.17), this difference was not statistically significant though.
Six months after ICU admission, BMD at the lumbar spine and femoral neck was not significantly different in the vitamin D group compared to the placebo group (Table 3). In the severe and less severe vitamin D deficiency subgroups, there was also no significant difference in BMD between the vitamin D and the placebo group. After 6 months, 42% (33/79) of patients were classified as osteopenic, and 13% (10/79) as osteoporotic by osteodensitometry.
Discussion
In this post hoc analysis of a prospective RCT in critically ill patients with 25-hydroxyvitamin D levels ≤20 ng/ml receiving high-dose vitamin D3 or placebo, we found no significant differences in bone turnover markers, BMD, fractures, and falls within 6 months after ICU admission between groups. Furthermore, we observed no significant effect of vitamin D supplementation on CTX and OC. There was, however, a significant increase of OC and a significant decrease of CTX after 6 months compared to baseline suppressed OC and elevated CTX in both study groups.
Besides vitamin D deficiency, other detrimental factors affecting skeletal health in this specific population include immobilization, rapid muscle loss during immobilization, inflammation, endocrine alterations, hypercatabolism, malnutrition, and drugs. All these factors likely contribute to increased bone resorption and accelerated bone loss as indicated by elevated CTX. Especially elderly ICU survivors also seem to carry an increased fracture risk, as suggested by the available limited clinical data [20]. This is highly relevant as fragility fractures, especially hip fractures, are deleterious events for an individual leading to decreased quality of life and often the loss of independence even in less vulnerable populations [27,28,29].
To overcome this situation of increased bone resorption and impaired skeletal health, Hollander and Mechanick suggested the consideration of intravenous bisphosphonates to potently decrease bone resorption [2]. They have been studied in small trials in critically ill adults and children and showed promising results [30,31,32]. Two recent retrospective observational studies suggested that preadmission bisphosphonate use in critically ill patients may even be associated with increased survival [33, 34].
The fact that no clear-cut difference was found in BMD after 6 months between patients receiving vitamin D and those receiving placebo might be attributed to the small sample size available for DXA after 6 months and the slow changes in BMD over time. Due to the nature of the investigated population, DXA scans were not performed at baseline. Furthermore, other factors such as immobilization and muscle loss as well as negative energy balances might have a stronger influence on BMD than vitamin D status alone. In view of the aging ICU population [35], the risk of critical illness-related falls and fractures could be substantial, an aggressive multidisciplinary approach may be indicated to reduce morbidity, mortality, and the high costs that have been documented in other, less sick populations [36,37,38].
Our data support the findings by Orford et al. [21], showing increased bone resorption during acute immobilization and improvement after recovery from critical illness. Similar to Orford, our patients showed a significant increase of OC after critical illness back to normal levels, but no commensurate response as a compensatory increase in bone formation.
In addition to limiting muscle loss by avoiding a negative energy balance, treatment of vitamin D deficiency with the aim to reach levels considered necessary for optimal bone health in other populations (above 20 ng/ml) [39] might be one easily adoptable treatment to improve skeletal consequences of prolonged critical illness. In the present study, however, we could not observe a significant effect of vitamin D supplementation on bone turnover markers. The development of suitable—currently unavailable—parenteral vitamin D monopreparations might contribute to more predictable increases in serum 25(OH)D levels [40], as critical illness-associated impaired gastrointestinal function or renal and drug-related compromises of the hepatic CYP450 system necessary for 25-hydroxylation of vitamin D might impair vitamin D uptake [41, 42].
There are important limitations to our study. These are the single center design, the lack of non-Caucasian or pediatric subjects, possibly limiting the generalizability of our findings and the relatively short follow-up for the outcome fractures, which were—as was the occurrence of falls—self-reported. Further, our trial was certainly underpowered for smaller effects, and spine X-rays detecting clinically silent vertebral fractures were not performed. Also, we did not evaluate how many patients were and were not able to ambulate before study inclusion and how many needed walking aids before and after the study, information that might be important regarding the impact of immobilization on bone turnover markers. Lacking information on nitrogen metabolism and balance and its association with bone turnover is another limitation of our study.
In conclusion, we observed increased bone resorption and decreased bone formation in critically ill patients with 25-hydroxyvitamin D levels ≤20 ng/ml, both conditions resolving over a follow-up period of 6 months. Vitamin D supplementation had no significant effect on CTX and OC. However, further studies are necessary to evaluate the relationship between vitamin D supplementation and BTM, BMD, and fracture risk in critically ill individuals with overt vitamin D deficiency. Perhaps a beneficial effect of vitamin D in critically ill patients may only be present in patients with very low vitamin D status, or a beneficial effect of vitamin D on bone health in critically ill patients might be entirely lacking.
References
Griffith DM, Walsh TS (2011) Bone loss during critical illness: a skeleton in the closet for the intensive care unit survivor? Crit Care Med 39:1554–1556
Hollander JM, Mechanick JI (2009) Bisphosphonates and metabolic bone disease in the ICU. Curr Opin Clin Nutr Metab Care 12:190–195
Via MA, Gallagher EJ, Mechanick JI (2010) Bone physiology and therapeutics in chronic critical illness. Ann N Y Acad Sci 1211:85–94
Rittweger J, Frost HM, Schiessl H, Ohshima H, Alkner B, Tesch P, Felsenberg D (2005) Muscle atrophy and bone loss after 90 days' bed rest and the effects of flywheel resistive exercise and pamidronate: results from the LTBR study. Bone 36:1019–1029
Gaudio A, Pennisi P, Bratengeier C, Torrisi V, Lindner B, Mangiafico RA, Pulvirenti I, Hawa G, Tringali G, Fiore CE (2010) Increased sclerostin serum levels associated with bone formation and resorption markers in patients with immobilization-induced bone loss. J Clin Endocrinol Metab 95:2248–2253
Van den Berghe G, Van Roosbroeck D, Vanhove P, Wouters PJ, De Pourcq L, Bouillon R (2003) Bone turnover in prolonged critical illness: effect of vitamin D. J Clin Endocrinol Metab 88:4623–4632
Vanhorebeek I, Langouche L, Van den Berghe G (2006) Endocrine aspects of acute and prolonged critical illness. Nat Clin Pract Endocrinol Metab 2:20–31
Kelly A, Levine MA (2013) Hypocalcemia in the critically ill patient. J Intensive Care Med 28:166–177
Fraser LA, Leslie WD, Targownik LE, Papaioannou A, Adachi JD, CaMos Research Group (2013) The effect of proton pump inhibitors on fracture risk: report from the Canadian multicenter osteoporosis study. Osteoporos Int 24:1161–1168
Veldhuis-Vlug AG, El Mahdiui M, Endert E, Heijboer AC, Fliers E, Bisschop PH (2012) Bone resorption is increased in pheochromocytoma patients and normalizes following adrenalectomy. J Clin Endocrinol Metab 97:E2093–E2097
Walsh JS, Newman C, Eastell R (2012) Heart drugs that affect bone. Trends Endocrinol Metab 23:163–168
Silverman SL, Lane NE (2009) Glucocorticoid-induced osteoporosis. Curr Osteoporos Rep 7:23–26
Kulak CA, Borba VZ, Kulak Junior J, Campos DJ, Shane E (2010) Post-transplantation osteoporosis. Arq Bras Endocrinol Metabol 54:143–149
Shane E, Rivas M, McMahon DJ, Staron RB, Silverberg SJ, Seibel MJ, Mancini D, Michler RE, Aaronson K, Addesso V, Lo SH (1997) Bone loss and turnover after cardiac transplantation. J Clin Endocrinol Metab 82:1497–1506
Nierman DM, Mechanick JI (1998) Bone hyperresorption is prevalent in chronically critically ill patients. Chest 114:1122–1128
Shapses SA, Weissman C, Seibel MJ, Chowdhury HA (1997) Urinary pyridinium cross-link excretion is increased in critically ill surgical patients. Crit Care Med 25:85–90
Smith LM, Cuthbertson B, Harvie J, Webster N, Robins S, Ralston SH (2002) Increased bone resorption in the critically ill: association with sepsis and increased nitric oxide production. Crit Care Med 30:837–840
Leblebici B, Sezgin N, Ulusan SN, Tarim AM, Akman MN, Haberal MA (2008) Bone loss during the acute stage following burn injury. J Burn Care Res 29:763–767
Muschitz GK, Schwabegger E, Kocijan R, Baierl A, Moussalli H, Fochtmann A, Nickl S, Tinhofer I, Haschka J, Resch H, Rath T, Pietschmann P, Muschitz C (2016) Early and sustained changes in bone metabolism after severe burn injury. J Clin Endocrinol Metab 101:1506–1515
Orford NR, Saunders K, Merriman E, Henry M, Pasco J, Stow P, Kotowicz M (2011) Skeletal morbidity among survivors of critical illness. Crit Care Med 39:1295–1300
Orford NR, Lane SE, Bailey M, Pasco JA, Cattigan C, Elderkin T, Brennan-Olsen SL, Bellomo R, Cooper DJ, Kotowicz MA (2016) Changes in bone mineral density in the year after critical illness. Am J Respir Crit Care Med 193:736–744
Schwetz V, Trummer C, Pandis M, Grubler MR, Verheyen N, Gaksch M, Zittermann A, Marz W, Aberer F, Lang A, Treiber G, Friedl C, Obermayer-Pietsch B, Pieber TR, Tomaschitz A, Pilz S (2017) Effects of vitamin D supplementation on bone turnover markers: a randomized controlled trial. Nutrients 9: https://doi.org/10.3390/nu9050432
Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, Nicholson GC (2010) Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA 303:1815–1822
EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) (2016) Dietary reference values for vitamin D. EFSA J 14(10):4547
Amrein K, Schnedl C, Holl A, Riedl R, Christopher KB, Pachler C, Urbanic Purkart T, Waltensdorfer A, Munch A, Warnkross H, Stojakovic T, Bisping E, Toller W, Smolle KH, Berghold A, Pieber TR, Dobnig H (2014) Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. JAMA 312:1520–1530
Amrein K, Schnedl C, Berghold A, Pieber TR, Dobnig H (2012) Correction of vitamin D deficiency in critically ill patients - VITdAL@ICU study protocol of a double-blind, placebo-controlled randomized clinical trial. BMC Endocr Disord 12:27,6823–12-27
Edwards BJ, Song J, Dunlop DD, Fink HA, Cauley JA (2010) Functional decline after incident wrist fractures--study of osteoporotic fractures: prospective cohort study. BMJ 341:c3324
Papaioannou A, Kennedy CC, Ioannidis G, Sawka A, Hopman WM, Pickard L, Brown JP, Josse RG, Kaiser S, Anastassiades T, Goltzman D, Papadimitropoulos M, Tenenhouse A, Prior JC, Olszynski WP, Adachi JD, CaMos Study Group (2009) The impact of incident fractures on health-related quality of life: 5 years of data from the Canadian multicentre osteoporosis study. Osteoporos Int 20:703–714
Pasco JA, Sanders KM, Hoekstra FM, Henry MJ, Nicholson GC, Kotowicz MA (2005) The human cost of fracture. Osteoporos Int 16:2046–2052
Klein GL, Wimalawansa SJ, Kulkarni G, Sherrard DJ, Sanford AP, Herndon DN (2005) The efficacy of acute administration of pamidronate on the conservation of bone mass following severe burn injury in children: a double-blind, randomized, controlled study. Osteoporos Int 16:631–635
Nierman DM, Mechanick JI (2000) Biochemical response to treatment of bone hyperresorption in chronically critically ill patients. Chest 118:761–766
Via MA, Potenza MV, Hollander J, Liu X, Peng Y, Li J, Sun L, Zaidi M, Mechanick JI (2012) Intravenous ibandronate acutely reduces bone hyperresorption in chronic critical illness. J Intensive Care Med 27:312–318
Lee P, Ng C, Slattery A, Nair P, Eisman JA, Center JR (2016) Preadmission bisphosphonate and mortality in critically ill patients. J Clin Endocrinol Metab 101:1945–1953
Schulman RC, Moshier EL, Rho L, Casey MF, Godbold JH, Zaidi M, Mechanick JI (2016) Intravenous Pamidronate is associated with reduced mortality in patients with chronic critical illness. Endocr Pract 22:799–808
Nguyen YL, Angus DC, Boumendil A, Guidet B (2011) The challenge of admitting the very elderly to intensive care. Ann intensive care 1:n5820-1-29
Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B, Oglesby AK (2003) The components of excess mortality after hip fracture. Bone 32:468–473
Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA (1999) Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet 353:878–882
Haentjens P, Magaziner J, Colon-Emeric CS, Vanderschueren D, Milisen K, Velkeniers B, Boonen S (2010) Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med 152:380–390
Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA (2011) The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 96:53–58
Vanek VW, Borum P, Buchman A, Fessler TA, Howard L, Jeejeebhoy K, Kochevar M, Shenkin A, Valentine CJ, Novel Nutrient Task Force, Parenteral Multi-Vitamin and Multi-Trace Element Working Group, American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors (2012) A.S.P.E.N. Position paper: recommendations for changes in commercially available parenteral multivitamin and multi-trace element products. Nutr Clin Pract 27:440–491
Spriet I, Meersseman W, de Hoon J, von Winckelmann S, Wilmer A, Willems L (2009) Mini-series: II. Clinical aspects. Clinically relevant CYP450-mediated drug interactions in the ICU. Intensive Care Med 35:603–612
Michaud J, Naud J, Ouimet D, Demers C, Petit JL, Leblond FA, Bonnardeaux A, Gascon-Barre M, Pichette V (2010) Reduced hepatic synthesis of calcidiol in uremia. J Am Soc Nephrol 21:1488–1497
Acknowledgements
Open access funding provided by Medical University of Graz. We would like to express our gratitude to the staff of the participating ICUs, to patients, relatives, family physicians, health care workers in other institutions and caregivers who made this trial possible. We also would like to acknowledge the support of Wolfgang Wolowszczuk for the sclerostin analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Karin Amrein and Harald Dobnig report grants from European Society for Clinical Nutrition and Metabolism (ESPEN), grants, lecture fees, and non-financial support from Fresenius Kabi (Germany), and a grant from the Austrian National Bank (Jubiläumsfonds) during the conduct of the study.
Conflicts of interest
None of the authors is in any regards financially dependent on the named institutions.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Schwetz, V., Schnedl, C., Urbanic-Purkart, T. et al. Effect of vitamin D3 on bone turnover markers in critical illness: post hoc analysis from the VITdAL-ICU study. Osteoporos Int 28, 3347–3354 (2017). https://doi.org/10.1007/s00198-017-4190-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-017-4190-1