Abstract
Introduction and hypothesis
A prospective clinical, preliminary study was performed in patients with interstitial cystitis/bladder pain syndrome (IC/BPS) who were nonresponders to conventional treatment and received intravesical ozone as a therapeutic alternative.
Methods
Sixteen patients received six applications of intravesical ozone at a concentration of 41 μg/mL. We evaluated therapeutic efficacy by the percentage reduction of Interstitial Cystitis Symptom and Problem Index scores (ICSI/ICPI—the O'Leary–Sant symptom index), recurrence rate, nonresponse, and side effects in scores collected on admission (pre-treatment), at the end of the therapeutic protocol (post-treatment), and 180 days (follow-up) after the last ozone application.
Results
The mean age of women was 52.9 years (SD: 15.5), and the duration of symptoms was 5.7 years (SD: 7.1). The median ICSI on admission was 17 (IQR: 14.25–19.5) and at follow-up was 0.5 (IQR: 0–2), with a reduction of 97.5% (CI: 85.7–100). The median ICSI/ICPI on admission was 31.5 (IQR: 29–35.2) and at follow-up was 2.0 (IQR: 0–3.75), with a reduction of 92.3% (CI: 88.8–100). The recurrence rate was only 6.25%, and no patients were nonresponders to the treatment.
Conclusions
The application of intravesical ozone was effective in the treatment of patients with IC/BPS who were nonresponders to conventional therapy, showing a progressive and safe effect, at least in the short term.
Similar content being viewed by others
Introduction
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a chronic, debilitating inflammatory disease with a negative impact on quality of life [1,2,3]. It is also defined as a sensation of pain, pressure, or discomfort related to the urinary bladder associated with urgency and frequency in the absence of infection or pelvic pathologies and lasting longer than 6 weeks [3,4,5]. The prevalence in women varies in the range of 2.7–6.5%, with symptoms usually starting after age 40, and increasing with age by up to 4.0% at age 80 years and over [4, 5, 7].
BPS is often associated with systemic somatoform disorders such as depression, fibromyalgia, and irritable bowel syndrome [2, 5,6,7]. The etiology is multivariate and not fully understood [6, 8, 9]. Alterations in urothelial tissue, neural hypersensitization, immunological mechanisms, and neurogenic inflammation are also involved in BPS [2]. BPS treatment has not been established, and there is no universally effective therapy [4, 8, 11]. The guidelines for pharmacotherapy are often limited, with a high rate of relapse, re-treatment, and side effects [10]. New and emerging IC/BPS therapies are needed, especially in patients with failure to respond to currently available treatments [8, 10, 11].
Ozone (O3) is an inorganic molecule with allotropic properties which consists of three oxygen atoms, and was identified by Schönbein in 1868 [12]. The interest in the therapeutic use of O3 (ozone therapy) is growing due to its anti-inflammatory, cytoprotective, antioxidant, antimicrobial, and immunomodulatory effects [13, 14].
The benefits of ozone therapy in pelvic and bladder inflammatory and infectious pathologies, in addition to a potential effect on renal parenchymal pathologies, have been evidenced in clinical and preclinical trials [13], for instance, the studies of Hidalgo-Tallón et al. and Tirelli et al. in fibromyalgia [15, 16], a long-term condition associated with urological disorders. In properly controlled doses, ozone triggers moderate and reversible oxidative stress with redox homeostasis and nitrosative stress, immune modulation, angiogenesis, vasodilation, and reduction of the inflammatory process, with a decrease in leukocytes and mast cells, as previously demonstrated in animal ozone models [17,18,19,20]. This, combined with its minimal side effects [13, 18,19,20], renders ozone a potentially effective therapeutic alternative for IC/BPS.
This study aimed to evaluate the effectiveness of intravesical administration of ozone as a complementary therapy for patients with IC/BPS nonresponsive to conventional treatment, alone or associated with oral and intravesical drug administration, using the O'Leary–Sant Interstitial Cystitis Symptom Index and Problem Index [21].
Materials and methods
Patients and study design
The protocol consisted of an experimental, prospective, longitudinal clinical study carried out in a group of female patients aged 25 years or older with IC/BPS who were nonresponders to behavioral treatment (first-line treatment) of IC, as well as at least two treatments established in the guidelines of the American Urological Association (AUA) [4, 5]. These treatments include oral medications (second-line treatments) alone or associated with intravesical drugs (third-line treatments) aimed at replenishing the layer of glycosaminoglycans with anti-inflammatory, analgesic, and muscle relaxant activity [8, 22].
We performed clinical diagnosis according to the criteria established by the European Society for the Study of Interstitial Cystitis (ESSIC) and by the O'Leary–Sant Interstitial Cystitis Symptom Index and Problem Index (ICSI/ICPI) (Table S1) [21]. The patients included in the study were required to have a score greater than 6 on the ICSI and the ICPI, with a total score (ICSI/ICPI) greater than 12. The ICSI/ICPI is validated as a measure of self-reported symptoms and perception of problems associated with IC/BPS [5, 23].
We excluded patients with the presence of sexually transmitted pathology or urinary tract infections (UTI) on admission, pregnant women, and those with bladder/uterine/cervical cancer. We also followed all contraindications for ozone administration proposed by the Madrid Declaration on Ozone Therapy [24].
The study experimental setup in the admission (period −1–0), treatment protocol (period 1–6), and the follow-up (period 7–9) is shown in Table 1.
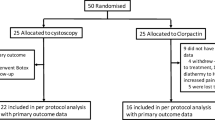
Thirty (n = 30) patients were recruited for the clinical study, and 16 (n = 16) were included in the study analysis. Figure 1 shows the study admission and the follow-up flow diagram in the CONSORT (Consolidated Standards of Reporting Trials) 2010 model.
The study was approved by the Research Ethics Committee of Anhembi Morumbi University (approval code CAAE 22106619.4.0000.5492) and previously registered in ClinicalTrials.gov (NCT04789135). All participants in the study signed an informed consent form (ICF). This study was conducted in accordance with the principles of the Declaration of Helsinki and followed the ethical standards of scientific research involving humans published by the Brazilian National Health Council (CNS), Resolution No. 466/12. Data collection was conducted from October 2019 to March 2021 at the patient care unit of the Distal Nephrology and Urology Association based in Jacareí, São Paulo, Brazil.
Ozone
Ozone was generated from medical-grade oxygen (99.5% O2) using electrical corona arc discharge by a photometric-calibrated O3 generator (MS3G, MS Instrumentos Industriais Ltda, Brazil). The oxygen flow rate supply at the input was adjusted to 0.125 L/min, with an ozone output concentration of 41 μg/mL, resulting in an ozone feed rate of 5.1 mg/min. The O2 + O3 mixture was generated at the administration site due to the ozone instability, and was promptly administered to the patient.
Gas administration was performed by an aseptic technique and with a disposable urinary catheter (no. 10; 3.3 mm diameter) until contact with the trigone of the urinary bladder. This urinary catheter was also connected to a silicon-treated polypropylene syringe (ozone-resistant) which received 60 mL of ozone at the ozone reactor outlet. Our pilot study previously determined this standardized volume (bladder capacity) following ozone therapy guidelines [24] and intravesical ozone protocols (50–100 mL) [13].
In each ozone therapy session, bladder lavage was performed, without hydrodistention, with 0.9% saline solution (500 mL, NaCl). After the lavage, bladder emptying was performed via a urinary catheter, followed by administration of a predetermined intravesical ozone gas (ozone mass of 2.5 mg) volume (60 mL) through a syringe connected to the urinary catheter. This volume was previously determined by uroflowmetry analysis (unpublished data) and data from other ozone guidelines [13, 24]. This application represents a potentially immunomodulatory and antioxidant stimulus effect [24] and a physiologically adequate level for systemic ozone administration, according to Viebahn-Hänsler and colleagues [25].
The patients, in standing position, were instructed to avoid the spontaneous liberation of the intravesical gas for 15 min. This time was mainly determined by the life of the ozone molecule (temperature dependency) [13]. The ozone applications were performed twice a week, with a total of six sessions (3 weeks total), also based on similar protocols [13, 24] and in an animal model study [17].
Evaluation approach
The ICSI/ICPI questionnaire was used to establish a symptom severity baseline at admission and to assess treatment response and IC/BPS relapse. The O'Leary–Sant ICSI/ICPI questionnaire [21] is composed of four questions on a Likert scale that generates scores for symptoms (ICSI), problems (ICPI), and total severity (ICSI/ICPI). The ICSI investigates aspects of urgency, frequency, nocturia, and pelvic pain, with an intensity ranging from 0 (not at all) to 5 (almost always). The ICPI assesses the impact attributed to symptoms from 0 (no problem) to 4 (major problem) [21]. The score is calculated by adding the point values for each item. In both indexes, a value greater than 6 or a total score greater than 12 is considered to indicate severe symptoms.
A gynecological exam was performed on all patients by Pap smear collection. In women with vaginitis, clinical samples were collected from the vaginal walls with a cotton-tipped swab and inoculated into tubes containing growth media (fresh exam, chocolate, blood, and MacConkey agar) for laboratory analysis. Gram smears were prepared and sent for examination according to the Nugent criteria. The material was analyzed for bacterial pathogens Chlamydia trachomatis, Ureaplasma, Neisseria gonorrhoeae, Trichomonas vaginalis, and Gardnerella vaginalis.
Urine samples collected were evaluated by physicochemical examination and microscopy. Hematuria was considered present if the erythrocyte count was above 5000/mL, and leukocyturia with a count greater than 8000 leukocytes/mL. Quantitative urine cultures (inoculation with MacConkey agar or eosin methylene blue and cystine–lactose–electrolyte-deficient [CLED] agar) were performed to exclude the presence of pathogenic microorganisms. The presence of urine cultures of pathogens above 100,000 colony-forming units (CFU)/mL (UTI condition) was considered an exclusion criterion. Abdominal and pelvic ultrasound exams of the urinary tract with a full bladder (uroflowmetry) were also performed (unpublished data).
The effectiveness of the ozone treatment was calculated based on the percentage difference between admission and the final score at the end of treatment by age group. We evaluated temporal effectiveness using the scores obtained during the 180-day follow-up period. Recurrence was defined as the return of symptoms, determined by ICSI ≥ 6 and ICSI/ICPI ≥ 12. Regarding the percentage of therapeutic effectiveness, we established a value below 50% as poor, moderate as 50–70%, good as 71–85%, and optimal response as values > 85%. A minimum of moderate (or 50%) improvement in patient response has become established in the medical community as a clinically relevant and significant improvement in IC/BPS [23]. A percentage of 100% indicates that the symptoms have disappeared, and each percentage value increment is considered a beneficial symptom improvement.
The presence of side effects possibly attributed to ozone use, such as a sensation of pain and burning, nausea, vomiting, lumbar pain, intestinal constipation, respiratory failure, headache, massive bleeding, or mild dyspnea, was monitored during ozone administration, between treatment sessions, and during follow-up (180 days).
Procedure-related events, such as urethral trauma, bleeding, and infectious conditions, were also evaluated. Data regarding menopause, genitourinary syndrome of menopause, vaginal atrophy, depression, irritable bowel syndrome, fibromyalgia, hematuria, and leukocyturia on admission were collected from the recent patient clinical history document.
Statistical analysis
We separate patients by age into four groups (27–40, 41–54, 55–68, 69–76 years) according to the evolutionary process of ovarian changes that begin at the age of 40 years, with a progressive reduction in estrogen and related to disorders in the genitourinary tract [26].
To assess the effect of ozone treatment on symptom scores, the measure of central tendency used included the median and dispersion, the quartiles, and interquartile range (IQR), with the results shown in percentages and confidence intervals (CI). We used the Kruskal–Wallis test as a statistical significance test, Dunn’s test as post hoc, and Spearman correlations between variables. The quantitative data are shown in absolute numbers, mean and standard deviation (SD).
The sample size (n = 30) was calculated using G*Power software (v. 3.1, Faul F., Germany) with an alpha (α) error of 0.05 (95%), effect size (ρ) of 0.53, and power of 0.8 (80%). Statistical analysis was performed using IBM SPSS Statistics software (v. 24.0, IBM Corp., Armonk, NY, USA) and OriginPro software (v. 8.5, OriginLab, Northampton, MA, USA). We define the null hypothesis rejection level at 0.05, with a 95% CI.
Results
In this work, 30 (n = 30) patients were recruited. Twelve (40%) of these were excluded due to UTI on admission. Eighteen (60%) met the eligibility criteria (allocation). In the follow-up period, two patients were lost to follow-up, one due to long COVID-19 illness and the other due to discomfort with using a urinary catheter. Thus, 16 (53.3%) patients were included in the analysis.
The mean patient age was 52.9 years (SD: 15.5 CI: 44.58–61.17), and the mean duration of symptoms was 5.7 years (SD: 7.1 CI: 1.52–9.95). There was no association between age and duration of symptoms (p = 0.06), although the mean symptom duration was longer in the older age group (69–74 years old). The mean age at onset of symptoms was 47.5 years (SD: 14.1), with 43.8% of patients experiencing symptom onset at an age between 34 and 47 years, 25% at 48–61 years, 18.8% at 20–33 years, and 12.5% at 62+ years (Table 2).
Historical strategies for patients with no response to therapeutic interventions included increased fluid intake, bladder training, and avoiding consumption of spicy foods. All patients also had prior interventions with pelvic physiotherapy and acupuncture therapy. Pharmacotherapy included amitriptyline (100 mg), solifenacin succinate (5 mg), oxybutynin (5 mg), doxazosin (2 mg), hydroxyzine (25 mg), and pentosan polysulfate (100 mg). Bladder instillation with heparin (5000 units), lidocaine (2%), and hydrocortisone (100 mg) was combined with oral medication.
Eight (50%) patients were in menopause, and of these, all had symptoms of genitourinary syndrome of menopause. In the oldest group, three (100%) patients had vaginal atrophy. Eleven (68.7%) patients reported depression, three (18.7%) had irritable bowel syndrome, and four (25%) had fibromyalgia. Urinary sediment analysis showed microscopic hematuria in four (25%) patients, and leukocyturia was observed in five (31.2%) patients (Table 3). Cystoscopy was performed on four patients (25%) with microscopic hematuria and a risk factor for bladder carcinoma; however, only one (6%) presented a Hunner lesion. The results were positive for Candida albicans in two (40%) samples, Candida krusei in one (16.6%), and Streptococcus agalactiae also in one (16.6%) individual, and one patient had a negative result.
The median ICSI/ICPI score on admission (pre-treatment) was 31.5 (IQR: 29–35.2), with an ICSI of 17 (IQR: 14.25–19.5) and an ICPI of 15.5 (IQR: 13–16). There was no correlation between the ICSI/ICPI scores with age (p = 0.66), symptom duration (p = 0.87), or age group (p = 0.09). At the end of the treatment protocol (post-treatment), the median ICSI/ICPI score was 6.5 (IQR: 2.25–11), representing a median reduction of 76.7% (CI 63.6–93.1), and the median ICSI was 4.0 (IQR: 2.0–5.5), with a reduction of 77.1% (CI: 64.7–87.5). The median ICSI/ICPI score at the end of follow-up (180 days) was 2.0 (IQR: 0–3.75), with a median reduction of 92.3% (CI: 88.8–100), and the median ICSI was 0.5 (IQR: 0–2.75), with a median reduction of 97.5% (CI: 85.7–100), representing a significant difference between admission and the end of follow-up (p < 0.001) (Table 4). Figure 2 presents a box plot showing the decrease in the ICSI score, the most important clinical O'Leary—Sant index.
At the end of the treatment protocol, four (25%) patients had ICSI/ICPI scores of 12 or greater, and three (75%) of these patients were aged 27–48 years. Median values were 17 (IQR: 13.2–21.5) for the total score and 8 (IQR: 6.5–11) for the ICSI. These values represented a median reduction of 47% (CI: 36.1–63.6) and 62.3% (CI: 45–64.7) between admission and post-treatment. The median ICSI/ICPI score at the 30-day follow-up was 9 (IQR: 3.25–9), representing a reduction of 80.6% (CI: 73.2–90.2), while the median ICSI score was 3.5 (IQR: 2.25–4.75), with a reduction of 82.5% (CI: 72.8–85.5) relative to the period of admission. At the end of 180 days, the median ICSI/ICPI score was 1.5 (IQR: 0.25–5.75), with a reduction of 95.1% (CI: 78.7–100), and a median ICSI score of 1.5 (IQR: 0.5–2.75), with a reduction of 90.3% (CI: 82.3–100).
At admission, the median score for urinary urgency and frequency was 5 (IQR: 4–5), and for pelvic pain, 5 (IQR: 5). No significant difference was found regarding the intensity of symptoms and age groups (p = 0.5). At the end of the treatment protocol, the median urinary urgency score was 1 (IQR: 0.25–1.0), with an 80% (CI: 66.6–100) reduction in symptom intensity and a significant difference between age groups (p = 0.002). Patients in the younger age group (27–40 years) had higher values, with a median of 2 (IQR: 2–3). The results for urinary frequency also showed a median of 1 (IQR: 1–1.75), with a reduction of 80% (CI: 40–80) but without a significant difference between age groups (p = 0.08). As for pelvic pain, the median was 0 (IQR: 0–1), with a 90% (CI: 50–100) reduction in symptom intensity and no significant difference between age groups (p = 0.66). At the end of the follow-up, 81.2% (13) of the patients had no urinary urgency, and 75% (12) had no pelvic pain. The results showed a significant difference (p < 0.001) between the admission (pre-treatment) and the follow-up periods (Table 5, Fig. 3).
No patients developed side effects attributed to ozone use. Nine (56.2%) patients had urethral discomfort. There was no urethral trauma, bleeding, or UTI in any period.
Discussion
IC/BPS is a chronic inflammatory process that affects the bladder wall. This process is characterized by neural hypersensitization, mast cell involvement [27], and the release of pro-inflammatory mediators such as histamine, serotonin, nitric oxide (NO), tumor necrosis factor (TNF-α), and activation of nuclear factor kappa beta (NF-κβ) [1, 7, 27].
This study presents, as a preliminary investigation, the results obtained with intravesical ozone at anti-inflammatory, immunomodulatory, and antioxidant dose [24]. The results showed a 76.7% reduction in the ICSI/ICPI score at the end of the therapeutic protocol and a 77.1% reduction in the ICSI. At the end of the follow-up period, the values reached 92.3% and 97.5%, respectively, representing an optimal therapeutic response and effectiveness over time, suggesting interruption of the inflammatory cycle associated with bladder changes that culminate in the clinical panorama of IC/BPS. At the end of the follow-up, 81.2% of patients reported no urinary urgency or pain, and 75% had no frequency control issues, representing a significant difference (p < 0.001) between pre-treatment and follow-up.
Bayrak et al. evaluated the effect of ozone in an animal model of IC and demonstrated that ozone has an anti-inflammatory effect on epithelial restoration and increased capillary angiogenesis [17]. That study agrees with the observations reported by Neimark et al. evaluating the use of ozone in patients with chronic cystitis through microcirculation parameters and vesical morphological changes. The authors demonstrated a restructuring of the epithelial layer, with structural organization, reduction of the inflammatory process, and significant improvement of microcirculation in the bladder mucosa [28]. These observations may explain our results confirmed through the ICSI/ICPI scores.
The mean age at onset of bladder symptoms in our study was 47.5 years, with at least 80% of patients experiencing onset of this clinical condition after 40 years of age. Sex hormones, especially estrogen, exert substantial effects on the female lower urinary tract [29]. The hormonal decline associated with menopause leads to histological and functional changes in the lower urinary tract, resulting in urogenital symptoms such as urinary urgency, frequency, and incontinence [26]. However, we did not observe a significant difference (p = 0.432) in the ICSI score between menopausal and non-menopausal patients.
Depression was found in 68.7% of patients, affecting 66.6% aged 27–40 and 100% aged 41–54 years. Watkins et al. reported a high prevalence of IC/BPS in patients with neurological and psychiatric disorders [30]. Depressive patients with IC/BPS report more pain than those without depression, and the coexistence of pain and depression makes the treatment more difficult, reduces recovery, increases the duration of symptoms, and predisposes patients to suicidal tendencies [31, 32]. Data obtained in the present study did not show a correlation between depression, maximum ICSI for pain on admission (p = 0.755), and the absence of therapeutic response (p = 0.755).
An initially moderate therapeutic response was observed in 25% of the patients, with a reduction of less than 50% in the ICSI/ICPI score and 62.3% in the ICSI. At the end of the follow-up, the ICSI reduction reached a value of 90.3% relative to admission, suggesting a progressive effect of intravesical ozone therapy not limited to the period of use, corroborating the recent results of Clavo et al. using ozone to treat chronic pelvic pain [33]. In this group, 75% of the patients were younger (27–40 years) and had a high prevalence of hematuria (66.6%). According to Quaghebeur and Wyndaele, hematuria has high sensitivity and specificity to differentiate ulcerative and non-ulcerative IC/BPS patients [34]; this response could suggest an ulcerative phenotype. Ulcerative IC/BPS presents intense epithelial denudation and chronic inflammatory changes, with compromised urothelial permeability and neuromodulated communication between the mucosa, the muscular layer, and the afferent neural system [1, 7]. High levels of NO related to NO synthase (inducible NO synthase [iNOS]) activity are also reported [35]. NO regulates several pathophysiological processes in inflammation, such as migration and adhesion of polymorphonuclear leukocytes.
Tasdemir et al. evaluated hemorrhagic cystitis caused by cyclophosphamide (CP) in an animal model and showed a significant increase in lipid peroxides and NO, and a reduction in glutathione (GSH). The study demonstrated that ozone co-treatment, especially 24 hours after using CP, prevented the changes with prominent NO depletion and GSH increases [18].
The effects associated with moderate and transient oxidative stress triggered by ozone include the upregulation of the antioxidant system, homeostasis of NO, calcium, and inflammatory cytokines, and improvement in oxygen metabolism and immune modulation, as reported in clinical and preclinical studies [15,16,17,18, 20, 28, 36, 37]. Adverse reactions to ozone are mild and transient, contributing to the growing interest in its use in various pathologies, including IC/BPS.
Cystoscopy was not conducted in our study as a diagnostic for IC/BPS, since it was not the objective to differentiate the clinical response to ozone treatment based on phenotype. Cystoscopy may be optional in young women with symptoms of IC/BPS and without risk factors for bladder cancer or other pelvic conditions [4]. The suspicion of phenotype with or without a Hunner lesion can be made through specific clinical characteristics that suggest the diagnosis [9].
The present study is a preliminary investigation employing an innovative approach that can be considered a proof-of-efficacy or single-arm intervention. The limitations of the study are its use of convenience sampling of a small group to evaluate the effectiveness of ozone therapy in patients with IC/BPS who were nonresponders to conventional treatment. New clinical investigations with a larger number of patients, through randomized clinical trials with a control and a sham group, and including histopathology, cystoscopy, urinary cytokines, biopsy, and molecular pathogen analysis patterns should be carried out, also aiming to determine the possible ozone mechanisms of action in IC/BPS, mainly due to the important appeal of patient nonresponders to conventional treatments.
Conclusion
The results presented herein, as a preliminary investigation, obtained through the O'Leary–Sant ICSI/ICPI scores showed a good, progressive response to ozone treatment, at least in the short term, and without side effects. The main findings of the current study suggest that intravesical ozone may contribute to the effective treatment of IC/BPS.
References
Akiyama Y, Luo Y, Hanno MP, et al. Interstitial cystitis/bladder pain syndrome: The evolving landscape, animal models and future perspectives. Int J Urol. 2020;27(6):491–503. https://doi.org/10.1111/iju.14229.
Birder LA. Pathophysiology of interstitial cystitis. Int J Urol. 2019;26(Suppl 1):12–5. https://doi.org/10.1111/iju.13985.
Malde S, Palmisani S, Al-Kaisy A, et al. Guideline of guidelines: bladder pain syndrome. BJU Int. 2018;122(5):729–43. https://doi.org/10.1111/bju.14399.
Cox A, Golda N, Nadeau G, et al. CUA guideline: Diagnosis and treatment of interstitial cystitis/bladder pain syndrome. Can Urol Assoc J. 2016;10(5-6):E136–55. https://doi.org/10.5489/cuaj.3786.
Hanno PM, Erickson D, Moldwin R, et al. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol. 2015;193(5):1545–53. https://doi.org/10.1016/j.juro.2015.01.086.
Huffman MM, Slack A, Hoke M. Bladder pain syndrome. Prim Care. 2019;46(2):213–21. https://doi.org/10.1016/j.pop.2019.02.002.
Patnaik SS, Laganà AS, Vitale SG, et al. Etiology, pathophysiology and biomarkers of interstitial cystitis/painful bladder syndrome. Arch Gynecol Obstet. 2017;295(6):1341–59. https://doi.org/10.1007/s00404-017-4364-2.
Garzon S, Laganà AS, Casarin J, et al. An update on treatment options for interstitial cystitis. Menopause Rev. 2020;19(1):35–43. https://doi.org/10.5114/pm.2020.95334.
Akiyama Y, Hanno MP. Phenotyping of interstitial cystitis/bladder pain syndrome. Int J Urol. 2019;1:17–9. https://doi.org/10.1111/iju.13969.
Giusto LL, Zahner PM, Shoskes DA. An evaluation of the pharmacotherapy for interstitial cystitis. Expert Opin Pharmacother. 2018;10:1097–108. https://doi.org/10.1080/14656566.2018.1491968.
Bosch PC. A randomized, double-blind, placebo-controlled trial of certolizumab pegol in women with refractory interstitial cystitis/bladder pain syndrome. Eur Urol. 2018;74(5):623–30. https://doi.org/10.1016/j.eururo.2018.07.026.
Sire A, Agostini F, Lippi L, et al. Oxygen-ozone therapy in the rehabilitation field: state of the art on mechanisms of action, safety and effectiveness in patients with musculoskeletal disorders. Biomolecules. 2021;11(3):356. https://doi.org/10.3390/biom11030356.
Bocci V. Ozone: A New Medical Drug. 2nd ed. Dordrecht: Springer; 2011. https://doi.org/10.1007/978-90-481-9234-2.
Di Mauro R, Cantarella G, Bernardini R, et al. The biochemical and pharmacological properties of ozone: the smell of protection in acute and chronic diseases. Int J Mol Sci. 2019;20(3):634. https://doi.org/10.3390/ijms20030634.
Hidalgo-Tallón J, Cepero SM, Vilchez JS, et al. Ozone therapy as add-on treatment in fibromyalgia management by rectal insufflation: An open-label pilot study. J Altern Complement Med. 2013;19(3):238–42. https://doi.org/10.1089/acm.2011.0739.
Tirelli U, Cirrito C, Pavanello M. Ozone therapy in 40 patients with fibromyalgia: an effective therapy. Ozone. Therapy. 2018;3(3). https://doi.org/10.4081/ozone.2018.7969.
Bayrak O, Erturhan S, Seckiner I, et al. Chemical cystitis developed in experimental animals model: Topical effect of intravesical ozone application to bladder. Urol Ann. 2014;6(2):122–6. https://doi.org/10.4103/0974-7796.130553.
Tasdemir S, Tasdemir C, Vardi N, et al. Effects of ozone therapy on cyclophosphamide-induced urinary bladder toxicity in rats. Clin Invest Med. 2013;36(1):E9–17. https://doi.org/10.25011/cim.v36i1.19400.
Teke K, Ozkan TA, Cebeci OO, et al. Preventive effect of intravesical ozone supplementation on n-methyl-n-nitrosourea-induced non-muscle invasive bladder cancer in male rats. Exp Anim. 2017;66(3):191–8. https://doi.org/10.1538/expanim.16-0093.
Wei A, Feng H, Jia X, et al. Ozone therapy ameliorates inflammation and endometrial injury in rats with pelvic inflammatory disease. Biomed Pharmacother. 2018;107:1418–25. https://doi.org/10.1016/j.biopha.2018.07.137.
O'Leary MP, Sant GR, Fowler FJ, et al. The interstitial cystitis symptom index and problem index. Urol. 1997;49(5):58–63. https://doi.org/10.1016/S0090-4295(99)80333-1.
Ogawa T, Ishizuka O, Ueda T, et al. Pharmacological management of interstitial cystitis /bladder pain syndrome and the role cyclosporine and other immunomodulating drugs play. Expert Rev Clin Pharmacol. 2018;11(5):495–505. https://doi.org/10.1080/17512433.2018.1457435.
Lubeck DP, Whitmore K, Sant GR, et al. Psychometric validation of the O’Leary-Sant interstitial cystitis symptom index in a clinical trial of pentosan polysulfate sodium. Urol. 2001;57(6 Suppl 1):62–6. https://doi.org/10.1016/s0090-4295(01)01126-8.
Schwartz A, Sánchez GM, Sabbah F et al. Madrid Declaration on Ozone Therapy. 3rd Ed. International Scientific Committee of Ozone Therapy. 2020. https://www.isco3.org. Accessed 10 May 2022.
Viebahn-Hänsler R, León Fernández OS, Fahmy Z. Ozone in medicine: The low-dose ozone concept—guidelines and treatment strategies. Ozone-Sci Eng. 2012;34(6):408–24. https://doi.org/10.1080/01919512.2012.717847.
Calleja-Agius J, Brincat MP. The urogenital system and the menopause. Climacteric. 2015;1:18–22. https://doi.org/10.3109/13697137.2015.1078206.
Shan H, Zhang EW, Zhang P, et al. Differential expression of histamine receptors in the bladder wall tissues of patients with bladder pain syndrome/ interstitial cystitis – significance in the responsiveness to antihistamine treatment and disease symptoms. BMC Urol. 2019;19(1):115. https://doi.org/10.1186/s12894-019-0548-3.
Neimark AI, Nepomnyashchikh LM, Lushnikova EL, et al. Microcirculation and structural reorganization of the bladder mucosa in chronic cystitis under conditions of ozone therapy. Bull Exp Biol Med. 2014;156(3):399–405. https://doi.org/10.1007/s10517-014-2358-7.
Hextall A, Cardozo L. The role of estrogen supplementation in lower urinary tract dysfunction. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(4):258–61. https://doi.org/10.1007/s001920170049.
Watkins KE, Eberhart N, Hilton L, et al. Depressive disorders and panic attacks in women with bladder pain syndrome/interstitial cystitis: A population-based sample. Gen Hosp Psychiatry. 2011;33(2):143–9. https://doi.org/10.1016/j.genhosppsych.2011.01.004.
McKernan LC, Bonnet KR, Finn MTM, et al. Qualitative analysis of treatment needs in interstitial cystitis/ bladder pain syndrome: Implications for intervention. Can J Pain. 2020;4(1):181–98. https://doi.org/10.1080/24740527.2020.1785854.
Tripp DA, Nickel JC, Krsmanovic A, et al. Depression and catastrophizing predict suicidal ideation in tertiary care patients with interstitial cystitis/bladder pain syndrome. Can Urol Assoc J. 2016;10(11-12):383–8. https://doi.org/10.5489/cuaj.3892.
Clavo B, Navarro M, Federico M, Borrelli E, et al. Long-term results with adjuvant ozone therapy in the management of chronic pelvic pain secondary to cancer treatment. Pain Med. 2021;22(9):2138–41. https://doi.org/10.1093/pm/pnaa459.
Quaghebeur J, Wyndaele J. Bladder pain syndrome (BPS): Symptom differences between type 3C BPS and non-type 3C BPS. Scand J Urol. 2015;49(4):319–20. https://doi.org/10.3109/21681805.2014.982170.
Logadottir Y, Hallsberg L, Fall M, et al. Bladder pain syndrome/interstitial cystitis ESSIC type 3C: High expression of inducible nitric oxide synthase in inflammatory cells. Scand J Urol. 2013;47(1):52–6. https://doi.org/10.3109/00365599.2012.699100.
Schulz S, Ninke S, Watzer B, et al. Ozone induces synthesis of systemic prostacyclin by cyclooxygenase-2 dependent mechanism in vivo. Biochem Pharmacol. 2012;83(4):506–13. https://doi.org/10.1016/j.bcp.2011.11.025.
Xing B, Chen H, Wang L, et al. Ozone oxidative preconditioning protects the rat kidney from reperfusion injury via modulation of the TLR4-NF-κB pathway. Acta Cir Bras. 2015;30(1):60–6. https://doi.org/10.1590/S0102-86502015001000008.
Acknowledgements
C. J. Lima, L. H. Moreira and A. B. Fernandes acknowledge the Ânima Institute (AI), Anhembi Morumbi University, São Paulo-SP, Brazil.
A. B. Fernandes thanks CNPq for the productivity fellowship (Process No. 310708/2021-4).
Financial Disclaimers
None.
Author information
Authors and Affiliations
Contributions
MV Pires: Protocol/project development, Data collection, Data analysis, Manuscript writing/editing.
CJ Lima: Protocol/project development, Manuscript writing/editing.
HC Carvalho: Protocol/project development, Data analysis, Manuscript writing/editing.
LH Moreira: Manuscript writing/editing.
AB Fernandes: Protocol/project development, Manuscript writing/editing.
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 17 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pires, M.V., de Lima, C.J., Carvalho, H.C. et al. Effectiveness of intravesical ozone in interstitial cystitis by the O'Leary–Sant symptom index. Int Urogynecol J 34, 1437–1446 (2023). https://doi.org/10.1007/s00192-022-05383-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-022-05383-3