Abstract
The development of additive manufacturing (AM) technologies has significantly advanced fabrication capabilities, yet achieving optimal surface quality and mechanical properties in end-use products is challenging. The primary objective of this study is to improve specific characteristics of 3D-printed components by employing a chemical post-processing technique including acetone. This technique is specifically applied to acrylonitrile butadiene styrene (ABS) material, utilizing a customized mechanical cold-vapor system. A complete investigation was undertaken to assess the effects of treatment on many factors, such as temperature, solvent volume, and exposure duration, on the tensile strength, physical dimensions, and mass of the ABS samples. Acetone post-processing has notably improved tensile strength, influenced by treatment duration and temperature and has led to dimensional changes such as a slight length reduction and increases in width and thickness. Furthermore, the mass of the samples exhibited variability upon acetone treatment, which was shown to be dependent on both the ambient temperature and the duration of solvent exposure. The tensile strength was assessed under various conditions, showing a significant enhancement at higher temperatures and longer exposure times. These results, demonstrating smoother surfaces and a tensile strength increase of up to 20% at 65 °C, underscore the efficacy of our techniques in modifying the mechanical and physical properties of 3D-printed ABS components. This innovative approach provides valuable insights into the relationship between post-processing conditions and ABS properties, enriching the body of knowledge in AM technology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Additive Manufacturing (AM) is a method that holds significant potential to produce complex geometries and customized parts, potentially offering cost-effectiveness and time efficiency. Its popularity has been steadily increasing over the past two decades [1, 2]. AM is a crucial component of contemporary manufacturing, playing a significant role in the progression of material science and engineering. It serves as a catalyst for advancements in product development and provides a transformational level of flexibility in production processes. In comparison to traditional manufacturing methods, AM also allows rapid prototyping and fabrication for end-user products. Consequently, intricate designs containing cavities and spiral holes can be efficiently produced. Prototypes manufactured using AM are semi-finished products with commendable mechanical features, making them suitable for evaluation as end-user products [3, 4]. Moreover, AM sub-techniques, including Fused Deposition Modeling (FDM), Binder Jetting (BJ), Material Jetting (MJ), Stereolithography (SLA), Laminate Object Manufacturing (LOM), and Powder Bed Fusion (PBF) [5], have become more accessible and affordable because of advancements in this area.
In the FDM technique, parts are constructed by depositing molten material layer by layer onto the construction platform, with Z-axis movement facilitated by the nozzle's motion [6]. The thermoplastic filament material is heated to a temperature close to its glass transition temperature (Tg) before being extruded from the nozzle. It subsequently cools and solidifies as it fuses with the previous layer [7]. The enhancement of the layer fusion mechanism is pivotal, as it significantly influences the mechanical strength and functional integrity of the printed structures. This fusion of materials during production results in favorable mechanical properties, which are subject to rigorous examination through post-production testing [8, 9]. FDM technology allows for the extrusion of various types of materials, including conventional thermoplastics such as ABS, PLA, and PC-ABS, as well as flexible thermoplastics like TPU and TPE [10]. Additionally, metals can also be used with FDM [11].
Acrylonitrile Butadiene Styrene (ABS) is a widely used extrudable thermoplastic material in FDM, with a usage rate of about 15%. ABS can fulfill various prototype requirements and is available in multiple color options [12]. Despite its extensive utilization, FDM production techniques often lead to suboptimal surface quality and diminished strength properties due to the inherent layering process. Consequently, post-processing is necessary to address this issue. One post-processing method used to improve surface quality is chemical post-treatment, which involves dissolving thermoplastic materials like ABS by interacting with chemical solvents (e.g., acetone, chloroform, methyl ethyl ketone) [13]. The necessity for this research is emphasized by the requirement to enhance the mechanical integrity and surface finish of FDM-produced ABS components, which are crucial for their applicability in practical scenarios.
Numerous studies have examined the effects of chemical post-treatment on the mechanical properties such as fracture behavior and surface quality of ABS samples produced via FDM [14, 15]. Chao et al. reported the use of chemical treatment to create a glossy coating layer on thermoplastics [16]. Another patent suggests the formation of a bright coating layer and an improvement in surface roughness of 3D-printed ABS through chemical post-treatment [17]. Addanki et al. investigated the mechanical effects of chemical post-treatment but did not conduct a comprehensive parameter sweep [18]. Garg et al. examined the impact of using acetone as a solvent in post-treatment on the enhancement of tensile and bending strength. The study noted anisotropic behavior and varying surface roughness depending on the production orientation [19]. Singh et al. reported a ~ 4% increase in surface hardness through hot acetone vapor treatment [20].
Conversely, Hambali et al. observed a 42.5% decrease in tensile strength and a 97.2% improvement in surface roughness when utilizing a dimethyl ketone and water solvent [21]. Jayanth et al. conducted a comparative analysis of acetone and dichloroethane solvents, revealing that acetone provided higher tensile strength while dichloroethane offered superior surface roughness [22]. The importance of a uniform temperature profile was studied by Mosavi et al. [23]. In a separate study, Mohammad Reza et al. achieved a 69% improvement in surface roughness through an immersion method, although the study did not consider the effects of ambient temperature and time [24]. Vishal et al. conducted a two-phase study to observe changes in surface hardness and roughness resulting from immersion in an acetone/silica nanoparticle solution and heat treatment [25]. Leonardo et al. utilized hot acetone vapor and found no significant alteration in surface roughness when varying distances between the treated surface and the acetone bath were considered. However, the test setup was confined to uniform parts [26]. Sotirios et al. investigated the antimicrobial effects of 3D printed PLA surfaces loaded with Ag and Cu against pathogens [27]. Lastly, there are different surface polishing approaches such as laser polishing [28, 29], electroplating [30], and vapor polishing [31].
This study establishes a benchmark in additive manufacturing by providing a novel, comprehensive analysis of cold acetone vapor treatment's effect on 3D printed ABS parts, setting a new standard for methodical post-treatment process control and detailed property enhancement evaluation. Herein, the ABS samples were subjected to different durations of chemical treatment (10, 20, 30, 40, and 50 min), varying ambient temperatures (30, 35, 45, 55, and 65 °C), and different amounts of total solvent (30, 50, and 70 mL). Additionally, the properties of ABS samples produced using FDM technology were evaluated at various vaporization speeds ranging from 0.6 to 7 mL/min. A custom-made mechanical cold-vapor setup was designed and utilized in this study to ensure experimental repeatability and accuracy. The impact of the proposed post-treatment on the physical properties (such as sample dimensions, volume, and mass), mechanical properties (tensile stress), and surface properties (smoothness) of the samples was examined. The study was divided into two phases: (i) identification of the optimal chemical solvent, where acetone exhibited the highest tensile stress of 16.36 MPa, and (ii) analysis of the effects of acetone under different conditions, including ambient temperature, treatment time, and solvent amount. The level of detail and breadth covered in this study positions it as a benchmark in the additive manufacturing industry and a catalyst for further innovative research in this domain.
2 Materials and methods
2.1 Sample fabrication
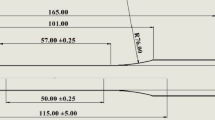
The 3D-printed sample design was completed using standard Computer-Aided Design (CAD) software (SolidWorks, Dassault Systems, France), adhering to ASTM D638 standards. Test samples were fabricated from natural color Acrylonitrile Butadiene Styrene (ABS) using commercial Ultrafuse (Ø1.75 mm, BASF, Germany). A Zortrax M200 printer (Zortrax, Poland) was employed, equipped with a Ø0.4 mm print nozzle, 0.19 mm layer thickness, and 20% infill (Fig. 1a). BASF ABS printing parameters included a nozzle temperature (Tn) of 260 °C and a bed temperature (TB) of 100 °C. To achieve tougher and smoother surfaces, the printing speed was reduced from the default 100 mm/s to 36 mm/s.
Overview of sample fabrication and investigation process. a Schematic representation depicting the process of sample production using a 3D printer. b Microscopic examination of sample layers, displaying the interlayer spacing in an untreated sample. c Microscope images illustrating the layer spacing for samples treated at different durations (0 to 50 min with a 10-min interval). Scale bars: 400 µm
2.2 Microscope inspection
The surfaces of the samples were inspected using a microscope from Soif (Shanghai Optical Instrument Co., China). All inspections were conducted using a built-in WF10x eyepiece microscope and 10 × zoom (Fig. 1b). Examination images were captured using an iPhone XS camera with an f/1.8 aperture, 1/122 s exposure time, ISO-32 ISO speed, 4 mm focal length, and without flash. The resulting images are 4032 × 3024 pixels in size, with a horizontal and vertical resolution of 72 dpi. Subsequently, the images were compared to each other to determine optimized experimental parameters (Fig. 1c), such as the cold vapor duration. As a result of chemical treatment, the interlayer spaces on the sample surface were filled, leading to a smoother surface in comparison to the untreated sample. We demonstrated that the gaps between the layers are completely closed when the processing time exceeds 20 min.
2.3 Chemical treatment
For the post-production chemical treatment, acetone (Sigma Aldrich, USA, purity ≥ 99.9%), MEK (Sigma Aldrich, USA, purity ≥ 99.9%), and chloroform (Sigma Aldrich, USA, purity ≥ 99.5%) were used as ABS samples dissolve in polar solvents. The samples underwent chemical treatment using the cold vapor method, facilitated by an atomizer located in the cartridge section of the test setup (Fig. 2a; Mov 1 and Mov 2, Supporting Information). This atomizer converted the solvent into cold vapor form, which was then transferred to the inner chamber using a pump. The samples were exposed to cold vapor at various temperatures (30, 35, 45, 55, and 65 °C), durations (10, 20, 30, 40, and 50 min), and total solvent volumes (30, 50, and 70 ml) to investigate the impact of these parameters on their mechanical properties and surface quality (Fig. 2b).
2.4 Experimental setup fabrication
The inner chamber and main body of the test setup were constructed using 1.5 mm thick 316L austenitic stainless steel (ASTM A240). The dimensions of the inner chamber are 200 mm × 200 mm × 200 mm (length x width x height), while the test setup measures 350 mm × 600 mm × 300 mm (Fig. 3). Tungsten Inert Gas (TIG) welding was employed to join the inner chamber, creating a fully enclosed environment that prevents the chemical gas from affecting the operator or surroundings. The sealing of the inner chamber was verified post-welding by conducting a water test, and impermeability was confirmed by filling the chamber with water and observing it for 24 h. To convert the chemical solvent from liquid to cold vapor form, an ultrasonic atomizer evaporator module (Yuansi, China) housed in a stainless-steel cartridge was utilized. The cold vapor was then transferred to the inner chamber using an Air Pump 200 (Eheim, China). A CNC machined Polytetrafluoroethylene (Teflon) pipe with high chemical resistance directed the steam into the inner chamber. To control the temperature of the inner chamber, a 120W MK2B heating plate with a built-in resistance of 4.8 ohms was employed. The test setup equipment was controlled using an Arduino Uno (Arduino LLC, USA), in addition to power supplies (5, 12, and 24 V) and electronic components such as a relay, switch, and power distributor. Further details can be found in Table S1 of the Supporting Information.
2.5 Mass measurement
Mass measurements were carried out using an AS220-R2 Plus balance (Radwag Balances and Scales, Poland), which has a range of 0 to 220 g and an accuracy of 0.1 µg. These measurements were conducted under identical experimental conditions, including temperature, time, total solvent volume, and solvent type (Fig. 4c). To analyze the effects of the coating layer formed after chemical interaction, the mass of the samples were compared with those of untreated samples.
Analyses were conducted on the samples as follows: a The tensile strengths of the samples were examined using a tensile test device, along with the assessment of stress-affected regions during the test. b Dimensional changes in the samples were observed after chemical treatment. c Sample masses were measured using precision scales
2.6 Tensile strength measurement
Tensile strength measurements were conducted using an Instron 8872 pulling device (Illinois Tool Works Inc., USA) (Fig. 4a; Fig. S1, Table S2, S3 Supporting Information). Chemically treated samples were attached to the handles of the device, aligning them with the longitudinal length of the sample. The experiment followed the standards for the 25 kN load cell device, employing a crosshead speed of 5 mm/min. The experiment was performed three times under identical conditions.
The following parametric approach was employed to analyze the data:
Maximum tensile stress calculation
The maximum tensile stress (σmax) is calculated using the formula:
where Fmax is the maximum load recorded (in Newtons) and A is the cross-sectional area of the specimen (in square millimeters).
Maximum tensile strain (Extension)
The maximum tensile strain (ϵmax) is determined as:
where ∆L is the change in length (in millimeters) and L0 is the initial length of the specimen (in millimeters).
Modulus of elasticity (E-modulus)
The modulus of elasticity (E) is calculated using the initial slope of the stress–strain curve:
where σelastic and ϵelastic are the stress and strain in the elastic region, respectively.
Yield stress and strain (0.2% offset method)
The yield stress (σyield) and strain (ϵyield) are determined using the 0.2% offset method. This involves drawing a line parallel to the initial linear portion of the stress–strain curve and offset by 0.2% strain. The intersection of this line with the curve gives the yield point.
Sample dimensions
The dimensions of each specimen (thickness, width, and length) are recorded to calculate the cross-sectional area and initial length, which are essential for the above calculations.
This parametric approach allows for the analysis of tensile strength and stress under varying conditions and for specimens with different dimensions. It provides a standardized method to interpret and compare the mechanical properties of the materials tested.
2.7 Dimensional measurement
After the chemical treatment, the outer dimensions of each sample (width x height x length) were measured as 20 × 115 × 4 mm, as shown in Fig. 4b. The measurements were conducted five times using an Insize Co. 1108–150 model digital vernier caliper from China. To compare and analyze the sample design dimensions, production dimensions, and chemically treated dimensions, an in-house developed program coded in MATLAB was utilized.
3 Results
This section presents the results of chemically treated ABS samples at various temperatures (30, 35, 45, 55, and 65 °C), durations (10, 20, 30, 40, and 50 min), total solvent volumes (30, 50, and 70 mL), and different solvent types (Acetone, MEK, Chloroform). The mechanical and surface properties, as well as the dimensional and mass changes of the samples, are reported by comparing the chemically treated samples with the untreated samples.
3.1 Tensile strength measurement
The elastic and plastic behavior of chemically treated and untreated samples under static loads up to 25 kN, with a crosshead speed of 5 mm/min, was investigated (Fig. 5). The samples underwent tensile testing in accordance with ASTM D638 standards. Tensile strength was analyzed as a function of temperature, time (i.e., duration of solvent exposure), total solvent amount, and solvent type, all under identical conditions.
The samples exhibited average tensile strengths of 16.36 MPa, 13.33 MPa, and 14.53 MPa for acetone, MEK, and chloroform, respectively (Fig. 5a). Based on these findings, acetone was chosen for the second phase due to its superior average strength in the initial phase. Subsequently, the samples were exposed to acetone cold vapor for varying durations, ranging from 10 to 50 min at 10-min intervals (Fig. 5b). The average maximum tensile strengths of the samples were as follows: 14.89 MPa (10 min), 13.89 MPa (20 min), 15.35 MPa (30 min), 15.20 MPa (40 min), and 14.53 MPa (50 min). Untreated ABS samples displayed an average maximum tensile strength of 16.85 MPa, which was higher compared to the chemically treated samples. The decrease in tensile strength was attributed to the solvent coating, as it modified the layer thickness of the bonding layer with the outer surface, resulting in reduced strength.
Different volumes of acetone solvent were applied to observe their effect on tensile strength at 30 mL, 50 mL, and 70 mL (Fig. 5c). By increasing the solvent volume, the total interaction between the cold vapor and the sample was enhanced. The effect of the coating structure formed due to chemical interaction on strength was observed, depending on the solvent volume. Furthermore, the impact of ambient temperature on the tensile strength and percent elongation of the samples was investigated (Fig. 5d). As the ambient temperature increased, the rate of chemical reaction accelerated, leading to faster coating formation on the sample. With the accelerated coating formation, the strength increased due to a deeper coating layer on the sample surface. The temperature increases also exhibited an annealing effect, resulting in the removal of internal stresses in the sample. The ductility of the chemically treated samples increased with the rise in ambient temperature. The average tensile strength was calculated as 15.35 MPa at 30 °C, and it increased by 32% (20.35 MPa) as the ambient temperature rose to 65 °C.
The observed decline in standard deviation as temperature increases implies a more consistent and pronounced impact of the solvent on the ABS samples at elevated temperatures. Nevertheless, the observed rise in variability at a temperature of 45 °C may suggest the existence of a distinct threshold at which the characteristics of the material experience substantial alterations.
3.2 Physical dimensions
Sample measurements were conducted on both chemically treated and untreated samples, which were then compared with the CAD dimensions of the samples. The CAD dimensions were recorded as 20 × 115 × 4 mm (Fig. 4b). After producing the samples at room temperature (25 °C), a length decrease of 0.03% (114.90 mm), a width increase of 0.9% (20.18 mm), and a thickness increase of 1.75% (4.07 mm) were observed.
3.2.1 Length
The length of the sample decreases as a function of temperature, time, and total solvent (Fig. 6). Among the various chemical solvents, chloroform provided the closest value to CAD measurements in terms of length. The coating formed during the chemical treatment involves the melting of the surface material as a result of the reaction, leading to a decrease in length (Fig. 6a). The sample treated for 50 min exhibited a length of approximately 0.30% less than that of the sample treated for 10 min (Fig. 6b). Increasing the total solvent volume from 30 to 70 mL resulted in a ~ 0.15% reduction in length (Fig. 6c). The length of the sample was reduced by ~ 1.03% when the ambient temperature increased from 30 °C to 65 °C (Fig. 6d). In addition, the observed trend of consistently low standard deviation across different temperatures and durations indicates that the chemical treatment procedure has a uniform impact on the length of ABS samples. The observed uniformity suggests that the solvent's interaction with the material remains consistent, hence ensuring the reliability of the procedure in managing sample dimensions within the measured parameters.
3.2.2 Width
The impact of solvent type on the width of ABS samples is demonstrated by the observed variations in the width (Fig. 7). Specifically, MEK exhibits a moderate drop in width, along with a greater standard deviation, as compared to acetone and chloroform (Fig. 7a). This observation implies the presence of distinct dynamics in the interaction between solvents and materials. Over a period of time, there is a noticeable upward trajectory in the width up to 40 min, suggesting enhanced penetration of the solvent and swelling of the polymer (Fig. 7b). This is subsequently followed by a slight decline at the 50-min point, coinciding with the highest standard deviation, which implies greater variability potentially caused by solvent saturation or evaporation.
When examining the influence of solvent volume on the dimensions of the ABS material, it is observed that the changes in width do not follow a linear pattern (Fig. 7c). This implies that there exists a certain threshold, beyond which the addition of more solvent does not result in further alterations in dimensions. This indicates the presence of a saturation point, when the ABS material reaches its maximum capacity for absorbing solvent. Finally, it is observed that the material experiences thermal effects, as evidenced by the increase in width from 20.02 mm at a temperature of 30 °C to a maximum of 20.34 mm at 55 °C (Fig 7d). Subsequently, there is a little reduction in width at 65 °C, which may suggest a potential limitation to thermal expansion or the initiation of solvent evaporation. The standard deviations observed at temperatures of 35 °C and 45 °C indicate a greater degree of variability in the thermal response of the material at these specific temperature levels. In the chemical processes conducted after 30 min, the material flowing for the coating, which began to solidify, led to the accumulation on the coating, consequently resulting in an increase in width. The observed limited standard variation in the width measurements suggests that the solvent treatment has consistently influenced the samples, resulting in the preservation of their dimensional stability.
3.2.3 Thickness
The examination of thickness in chemically treated ABS samples reveals discernible reactions to several types of solvents. The application of acetone at a temperature of 25 °C for a duration of 30 min consistently yielded a thickness measurement of 4.07 mm. This observation is supported by the minimal standard deviation, indicating that acetone's effect on thickness is generally constant and uniform. In contrast, the application of chloroform resulted in a more substantial augmentation in thickness, reaching a maximum value of 4.14 mm. The higher standard deviation associated with this treatment indicates a less consistent impact observed throughout the many samples. This variability with chloroform could be due to its aggressive solvation properties causing significant swelling, whereas acetone's interaction with ABS appears to be more controlled and efficient, yielding a more desirable and consistent treatment result. On the other hand, observed samples exhibited a consistent growth in thickness, with measurements indicating a rise from 4.07 mm at the 10-min mark to 4.13 mm at the 50-min mark, while being subjected to a constant temperature of 30 °C (Fig. 8b). This trend suggests a temporal influence of the solvent on the ABS material. The observed minor standard deviations over this timeframe, namely 0.01 at the beginning and 0.05 at the conclusion, suggest a continuous trend in the process of solvent absorption and material softening over time (Fig. 8b).
The correlation between the volume of solvent and the thickness of the sample is apparent. Specifically, when the solvent volume is increased from 30 to 70 mL, there is not a linear rise in thickness (Fig. 8c). Instead, there is a mild increase from 4.07 mm to 4.21 mm. The observed tiny standard deviations, namely 0.01 for the 30 mL and 0.02 for the 70 mL, indicate that an increase in solvent quantity contributes to an increase in thickness. However, there appears to be a saturation point where further addition of solvent yields diminishing returns. The significance of temperature is of utmost importance since the thickness exhibits a gradual rise throughout the temperature range of 30 °C to 65 °C. At a temperature of 30 °C, the initial thickness is measured to be 4.07 mm, which subsequently increases to 4.21 mm at 65 °C (Fig. 8d). This observation suggests that elevated temperatures enhance solvent activity and encourage the growth of the polymer material. It is worth mentioning that there is a marginal increase in the standard deviation from 0.01 to 0.02 as the temperature increases. This finding further supports the notion that temperature consistently and reliably affects the thickness of treated ABS samples.
3.2.4 Volume
Regarding the influence of solvent type on the volume of ABS samples, the experimental data derived from treatments with acetone, MEK, and chloroform under fixed temperature and duration conditions, demonstrate that the solvent choice exerts a significant effect on volume changes (Fig. 9a). Chloroform treatment resulted in the highest increase in volume, whereas MEK was associated with the least volumetric change. This variation could be attributed to the different solvation strengths and diffusion rates of each solvent into the ABS matrix. In examining the impact of time on volume, the results showed variability, with the highest volume recorded after a treatment duration of 50 min (Fig. 9b). This trend suggests a cumulative effect of solvent absorption over time, yet this relationship does not appear to be strictly linear, as evidenced by a slight decrease in volume at the 30-min mark, followed by increases at subsequent 40 and 50-min intervals. The non-linear volume changes could be due to the dynamic balance between solvent penetration and evaporation over the course of treatment.
Furthermore, the escalation in volume with increasing amounts of solvent from 30 to 70 mL, while other conditions remained constant, underlines the dose-dependent nature of the solvent's impact on the volume of polymer (Fig. 9c). Upon a thorough analysis of the data, it is apparent that temperature significantly influences the volume of acetone-treated ABS samples (Fig. 9d). Measurements revealed a pattern of increasing volume with rising temperature from 30 °C to a peak at 55 °C, followed by a slight decrease at 65 °C. The thermal expansion at elevated temperatures is likely a result of increased solvent activity causing swelling of the polymer matrix. However, the reduction in volume at 65 °C, which deviates from the trend, may indicate that solvent evaporation is prevailing over the swelling effect, or there may be a reorganization of polymer chains as they near a state of greater thermal relaxation. Notably, elevated standard deviations at 35 °C and 45 °C suggest increased variability in sample response at these temperatures, which may be due to complex interactions between solvent absorption, polymer expansion, and the beginning of evaporation.
3.3 Mass analysis
After conducting an analysis of the mass variations in ABS samples following chemical treatment, the initial mass was measured at 5.0489 gr by utilizing untreated samples as a control group. The choice of solvent employed was a critical factor, as evidenced by the significant mass increase of 6.93% (5.4031 g) observed with chloroform treatment, indicating a strong solvation interaction (Fig. 10a). Conversely, MEK treatment resulted in the lowest increase of 2.38% (5.1664 g), suggesting a weaker interaction. Acetone treatment yielded a moderate increase of 2.97% (5.2018 g). Over the course of the experiment, the mass of the samples exhibited a gradual increase, starting with a rise of 0.99% within a span of 10 min and culminating in a 4.95% increase after 50 min (Fig. 10b). This observed trend may be attributed to the complex interplay between solvent absorption, chemical reaction rates, and evaporation. In addition, it should be noted that the mass was found to be impacted by the total amount of solvent employed. Specifically, an escalation in solvent volume from 30 to 70 mL resulted in an increase in mass from 1.98% to 3.56% (Fig. 10c). This observation underscores the dose-dependent characteristic of the solvent's effect. The influence of ambient temperature was also seen, with the mass exhibiting a significant rise from 2.57% at 30 °C to 5.74% at 65 °C (Fig. 10d). This observation suggests that elevated temperatures may either facilitate solvent absorption or expedite chemical processes, hence impacting the overall mass increment. The observed standard deviations among these variables suggest that there are more intricate relationships at higher temperatures, possibly attributed to accelerated absorption rates and thermally induced processes.
4 Discussion
This study thoroughly investigates the impact of polar chemical solvents on the mechanical properties, surface characteristics, dimensional changes, and mass of samples produced using additive manufacturing methods. A custom-designed test setup was employed to examine the effects, considering various parameters such as ambient temperatures, processing times, solvent amounts, and types of solvents. The key findings are summarized below:
-
Acetone chemical treatment has demonstrated a considerable increase in mechanical strength, 12.59% more than chloroform and 22.73% more than MEK. This increase is attributed to the higher polarity of acetone, which accelerates the reaction rate and enhances bond structures.
-
The samples treated with acetone have exhibited a decline in mechanical strength ranging from a maximum of 17.56% to a minimum of 8.9%, compared to the untreated samples. This decline is primarily due to the residual stress introduced by the chemical treatment. The decrease in strength is attributed to the notch effect caused by gaps between layers and the increase in chemically reacting material after 30 min of treatment.
-
Increasing the solvent amount from 30 to 70 mL has led to higher vapor density, resulting in stable dissolution on the sample. However, this effect may vary for samples with different surface areas. Raising the ambient temperature from 25 °C to 65 °C caused a softening of the internal structure of the ABS samples, resulting in high viscosity yields. This has led to a 1.03% decrease in length and a 3.69% increase in thickness, which improved layer adhesion and boosted mechanical strength by 32% at 65 °C.
-
Chemical absorption during treatment has increased the samples' mass by up to 5.73%, with a direct proportion to temperature, time, and solvent amount. Notably, acetone-treated samples at 30 °C for 50 min and 65 °C for 30 min showed 4.81% and 5.73% mass increases, respectively, compared to untreated samples.
These results can help optimize post-processing techniques for additive manufacturing products. The significant influence of acetone treatment on ABS-printed parts has been demonstrated, prompting an exploration of other chemical solvents that may have different, potentially more beneficial interactions with 3D-printed components. Moreover, the residual stress introduced by the chemical treatment warrants a deeper understanding, with future studies potentially focused on ways to minimize this stress while retaining the enhanced mechanical strength.
5 Conclusions
Through meticulous study design, transformative insights have been uncovered that will aid in the furtherance of additive manufacturing and the optimization of post-processing techniques. This work addresses a significant knowledge gap by elaborating on the nuanced influence of chemical solvents, particularly acetone, on mechanical properties, surface characteristics, dimensional attributes, and mass changes of 3D-printed parts.
-
The examination of various chemical treatments applied to Acrylonitrile Butadiene Styrene (ABS) samples yields significant improvements in mechanical properties, surface characteristics, dimensional stability, and mass. The present study utilized a range of solvents, temperatures, treatment durations, and solvent volumes, with a particular focus on material exploited in the field of additive manufacturing.
-
Acetone has shown superior efficacy in enhancing mechanical strength compared to chloroform and Methyl Ethyl Ketone (MEK) within the investigated solvents. The observed improvement is attributed to the increased polarity of acetone, which facilitates the acceleration of reaction rates and the reinforcement of binding structures inside ABS.
-
One interesting observation is the decrease in mechanical integrity observed in samples treated with acetone in comparison to those that were not treated. This phenomenon is expected to be caused by the presence of residual stresses resulting from the chemical treatment. The mentioned stresses have a negative effect on the structural integrity, especially when subjected to extended periods of exposure.
-
A greater amount of solvent volume resulted in enhanced uniformity of dissolution processes, but elevated ambient temperatures had a weakening effect on the internal structure of ABS. The process of softening had a role in enhancing the bonding between layers, resulting in a significant enhancement in the mechanical strength of the material when exposed to high temperatures.
-
The samples exhibited a rise in mass as a consequence of chemical absorption during the treatment process, with a direct correlation observed between this increase in mass and the variables comprising temperature, time, and solvent volume. The samples that were treated with acetone demonstrated significant increases in mass under specified conditions, emphasizing the influence of acetone on the properties of the material.
-
The efficacy of utilizing acetone treatment on ABS components presents opportunities for further investigation into alternative chemical solvents and their interactions with materials produced by 3D printing technology. In addition, it is imperative to consider the issue of residual stress caused by chemical treatments in order to uphold improved mechanical characteristics and material integrity.
-
Future research could explore the integration of non-contact, thermal laser polishing methods for a more comprehensive approach to enhancing surface quality with chemical solvent treatments in 3D-printed components [32]. Additionally, future investigations could investigate how biological processes can be integrated into material processing, aiming to develop eco-friendly and sustainable manufacturing practices [33].
In conclusion, this research offers enlightening revelations that not only enhance the understanding of post-processing techniques but also open new horizons in the additive manufacturing landscape. By harnessing these findings, strides can be made toward refining the manufacturing process, improving product quality, and optimizing performance, thus propelling additive manufacturing technology into a promising future.
References
Pegues J, Roach M, Scott Williamson R, Shamsaei N (2018) Surface roughness effects on the fatigue strength of additively manufactured ti-6Al-4V. Int J Fatigue 116:543–552. https://doi.org/10.1016/j.ijfatigue.2018.07.013
Chueca de Bruijn A, Gómez-Gras G, Pérez MA (2021) A comparative analysis of chemical, thermal, and mechanical post-process of fused filament fabricated polyetherimide parts for surface quality enhancement. Materials 14:5880. https://doi.org/10.3390/ma14195880
Kumbhar NN, Mulay AV (2016) Post processing methods used to improve surface finish of products which are manufactured by additive manufacturing technologies: a review. J Inst Eng (India): Ser C 99:481–487. https://doi.org/10.1007/s40032-016-0340-z
Chockalingam K, Jawahar N, Praveen J (2015) Enhancement of anisotropic strength of fused deposited ABS parts by genetic algorithm. Mater Manuf Process 31:2001–2010. https://doi.org/10.1080/10426914.2015.1127949
Singh R, Singh S, Singh IP, Fabbrocino F, Fraternali F (2017) Investigation for surface finish improvement of FDM parts by vapor smoothing process. Compos B Eng 111:228–234. https://doi.org/10.1016/j.compositesb.2016.11.062
Del Sol I, Domínguez Calvo Á, Piñero D, Salguero J, Batista M (2019) Study of the FDM parameters of the ABS parts in the surface quality after machining operations. Key Eng Mater 813:203–208. https://doi.org/10.4028/www.scientific.net/kem.813.203
Iftikhar A, Khan M, Alam K, Imran Jaffery SH, Ali L, Ayaz Y, Khan A (2013) Turbine blade manufacturing through rapid tooling (RT) process and its quality inspection. Mater Manuf Process 28:534–538. https://doi.org/10.1080/10426914.2012.746698
Gurrala PK, Regalla SP (2014) Part strength evolution with bonding between filaments in fused deposition modelling. Virtual Phys Prototyp 9:141–149. https://doi.org/10.1080/17452759.2014.913400
Chohan JS, Singh R, Boparai KS (2016) Mathematical modelling of surface roughness for vapour processing of ABS parts fabricated with fused deposition modelling. J Manuf Process 24:161–169. https://doi.org/10.1016/j.jmapro.2016.09.002
Cho K-J, Koh J-S, Kim S, Chu W-S, Hong Y, Ahn S-H (2009) Review of manufacturing processes for soft biomimetic robots. Int J Precis Eng Manuf 10:171–181. https://doi.org/10.1007/s12541-009-0064-6
Kim M-S, Chu W-S, Kim Y-M, Avila APG, Ahn S-H (2009) Direct metal printing of 3D electrical circuit using rapid prototyping. Int J Precis Eng Manuf 10:147–150. https://doi.org/10.1007/s12541-009-0106-0
Percoco G, Lavecchia F, Galantucci L (2012) Compressive properties of FDM rapid prototypes treated with a low cost chemical finishing. Res J Appl Sci Eng Technol 4:3838–3842
Khosravani MR, Anders D, Reinicke T (2023) Effects of post-processing on the fracture behavior of surface-treated 3D-printed parts. CIRP J Manuf Sci Technol 46:148–156. https://doi.org/10.1016/j.cirpj.2023.08.006
Hambali RH, Cheong KM, Azizan N (2017) Analysis of the influence of chemical treatment to the strength and surface roughness of FDM. IOP Conf Ser: Mater Sci Eng 210:012063. https://doi.org/10.1088/1757-899x/210/1/012063
Mishra SB, Acharya E, Banerjee D, Khan MS (2019) An experimental investigation of surface roughness of FDM build parts by chemical misting. IOP Conf Ser: Mater Sci Eng 653:012043. https://doi.org/10.1088/1757-899x/653/1/012043
Google Patents. Available: https://patents.google.com/patent/US4247580A/en. Accessed 2 Oct 2022
Google Patents. Available: https://patents.google.com/patent/US8123999B2/en. Accessed 2 Oct 2022
Rao AS, Dharap MA, Venkatesh JVL (2015) Experimental study of the effect of post processing techniques on mechanical properties of fused deposition modelled parts. Int J Manuf Mater Mech Eng 5:1–20. https://doi.org/10.4018/ijmmme.2015010101
Garg A, Bhattacharya A, Batish A (2016) Chemical vapor treatment of ABS parts built by FDM: analysis of surface finish and mechanical strength. Int J Adv Manuf Technol 89:2175–2191. https://doi.org/10.1007/s00170-016-9257-1
Singh R, Singh S, Singh IP (2016) Effect of hot vapor smoothing process on surface hardness of fused deposition modeling parts. 3D Print Addit Manuf 3:128–133. https://doi.org/10.1089/3dp.2016.0001
Hambali HR, Cheong MK, Azizan N (2017) Analysis of the influence of chemical treatment to the strength and surface roughness of FDM. IOP Conf Ser Mater Sci Eng 210:012063. https://doi.org/10.1088/1757-899X/210/1/012063
Jayanth N, Senthil P, Prakash C (2018) Effect of chemical treatment on tensile strength and surface roughness of 3D-printed ABS using the FDM process. Virtual Phys Prototyp 13:155–163. https://doi.org/10.1080/17452759.2018.1449565
Mosavi A, Salehi F, Nadai L, Karoly S, Gorji NE (2020) Modeling the temperature distribution during laser hardening process. Results Phys 16:102883. https://doi.org/10.1016/j.cirpj.2023.08.006
Khosravani MR, Schüürmann J, Berto F, Reinicke T (2021) On the post-processing of 3D-printed ABS parts. Polymers 13(10):1559. https://doi.org/10.3390/polym13101559
Francis V, Garg S, Saxena KK, Jain PK, Lade J, Kumar D (2022) Effect of chemical and heat treatment on 3D printed parts: nanoparticles embedment approach. Adv Mater Process Technol:1–12. https://doi.org/10.1080/2374068x.2022.2037876
Riva L, Fiorentino A, Ceretti E (2022) Characterization of chemical surface finishing with hot acetone vapours on ABS parts fabricated by FFF. Prog Addit Manuf. https://doi.org/10.1007/s40964-022-00265-y
Εkonomou SΙ, Soe S, Stratakos AC (2023) An explorative study on the antimicrobial effects and mechanical properties of 3D printed PLA and TPU surfaces loaded with Ag and Cu against nosocomial and foodborne pathogens. J Mech Behav Biomed Mater 137:105536. https://doi.org/10.1016/j.jmbbm.2022.105536
Arthanari S, Park JE, Heo JS, Cho DH, Yang M, Hwang JS, Lee H (2023) Laser surface polishing of 3D printed polylactic acid (PLA) with different levels of absorption. J Manuf Process 98:265–276. https://doi.org/10.1016/j.jmapro.2023.05.034
Mushtaq RT, Iqbal A, Wang Y, Khan AM, Petra MI (2023) Advancing PLA 3D printing with laser polishing: improving mechanical strength, sustainability, and surface quality. Crystals 13(4):626. https://doi.org/10.3390/cryst13040626
Eßbach C, Fischer D, Nickel D (2021) Challenges in electroplating of additive manufactured ABS plastics. J Manuf Process 68:1378–1386. https://doi.org/10.1016/j.jmapro.2021.06.037
Neff C, Trapuzzano M, Crane NB (2016) Impact of vapor polishing on surface roughness and mechanical properties for 3D printed ABS. In: 2016 International Solid Freeform Fabrication Symposium. University of Texas at Austin
Ukar E, Lamikiz A, Martínez S, Tabernero I, De Lacalle LL (2012) Roughness prediction on laser polished surfaces. J Mater Process Technol 212(6):1305–1313. https://doi.org/10.1016/j.jmatprotec.2012.01.007
Díaz-Tena E, Rodríguez-Ezquerro A, de Lacalle Marcaide LL, Bustinduy LG, Sáenz AE (2014) A sustainable process for material removal on pure copper by use of extremophile bacteria. J Clean Prod 84:752–760. https://doi.org/10.1016/j.jclepro.2014.01.061
Acknowledgements
Abdurrahim Yilmaz has been funded by the President’s PhD Scholarships of Imperial College London. This study was funded by the Yildiz Technical University Scientific Research and Project Coordination Unit under the Guided Project numbered FBG-2023-5411.
Author information
Authors and Affiliations
Contributions
Ali Anil Demircali: Formal Analysis, Investigation, Data Curation, Writing – original draft preparation, Writing – review and editing, Visualization. Durmus Yilmaz: Conceptualization, Methodology, Experimental Studies, Writing – original draft preparation. Abdurrahim Yilmaz: Validation, Writing – original draft preparation, Writing – review and editing. Onur Keskin: Methodology, Funding Acquisition. Meysam Keshavarz: Validation, Writing – review and editing, Supervision. Huseyin Uvet: Validation, Resources, Supervision, Project Administration, Funding Acquisition.
All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MOV 5006 kb)
Supplementary file3 (MOV 4912 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Demircali, A.A., Yilmaz, D., Yilmaz, A. et al. Enhancing mechanical properties and surface quality of FDM-printed ABS: A comprehensive study on cold acetone vapor treatment. Int J Adv Manuf Technol 130, 4027–4039 (2024). https://doi.org/10.1007/s00170-023-12929-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00170-023-12929-2