Abstract
The article presents the results of research on the ion nitriding process of the so-called difficult to nitride 316L austenitic steel. The main aim of these studies was to determine what influences the intensification of the nitriding process as a result of using an active screen. Two variants of placing the nitrided elements in the glow discharge chamber were adopted: directly on the cathode and on the cathode using an active screen. After the nitriding processes were carried out, the influence of the adopted process parameters on the depth of nitrogen diffusion into the nitrided substrate was analyzed. In the further part of the work, an ionizing nitriding mechanism with the active screen method was proposed, explaining the significant increase in the effectiveness of the nitriding process using the active screen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The dynamic development of modern science and technology makes it necessary to use materials with increasingly favorable mechanical properties, in particular fatigue strength, corrosion resistance, and wear in conditions of friction. These basic features depend on the properties of the appropriately constituted surface layer of these elements. In modern engineering practice, the applied surface layers, with different chemical and phase compositions as well as morphology of the phase components of the microstructure, are produced in heat and thermo-chemical treatment processes.
The currently used metallic materials do not often meet the requirements related to their intended use. Innovative surface engineering technologies create new opportunities in shaping their microstructure to provide them specific mechanical and functional properties. Most research works focus on modifying the chemical and phase composition of metallic materials or their surfaces in order to create surface layers that meet high quality requirements [1,2,3].
Nitriding as one of the types of thermo-chemical treatment currently belongs to the group of the fastest developing methods of surface engineering. This process makes it possible to improve the mechanical and functional properties of structural elements and machine parts, above all their hardness and strength and resistance to wear in conditions of friction as well as corrosion resistance. The current stream of scientific and utilitarian research concerns phase changes in iron nitrides occurring during the nitriding process of both solid and powdered metallic materials [4,5,6].
Iron nitrides are metastable phases at atmospheric pressure; the stability of iron nitrides at higher temperatures requires a physical N2 pressure of several gigapascals. A quasi equilibrium state can be obtained chemically at atmospheric pressure using a gas mixture NH3/H2 [7]. Numerous studies have shown that the thermal stability of the iron nitride phase composition depends on the form and size of iron nitrides (thin layers, foils, micropowders, nanopowders, etc.) [8] and heating conditions [9]. The results of theoretical and experimental work relate primarily to the association of the thermal stability of iron nitrides with their magnetic properties [10, 11].
The nitriding process of high-chromium steels is associated with great difficulty in its implementation due to the presence of an airtight layer of chromium oxides on the surface of these steels blocking the penetration of nitrogen into the material. For this reason, in order to activate the surface in the gas nitriding process of these steels, etching is carried out in industrial practice by introducing active gases into the nitriding atmosphere (e.g., NH4Cl or HCl) producing active coatings (e.g., phosphate, nickel, or sulfur coatings) grinding, or polishing; pre-ion treatment (cathodic sputtering) in ion treatment equipment with subsequent transfer of the pre-processed elements to the furnace in which the main gas nitriding process is carried out [4, 12, 13].
Plasma nitriding is a surface treatment widely used in the industry to improve the hardness, wear resistance, and in some cases the corrosion resistance of steels. The nitriding process in glow discharge conditions allows the production of layers of better quality than after conventional gas nitriding. In addition, ion nitriding enables the removal of oxide layers that impede the diffusion of nitrogen, already in the initial stage of this process, while eliminating the need to pre-activate the surface [14]. Activation of the surface by cathodic sputtering is combined with bombardment with low-energy ions. The energy value of these ions must be greater than the energy threshold values for sputtering surface atoms [15]. According to the authors of papers [16,17,18,19,20], conventional nitriding methods are conducted at temperatures above 500 °C, which causes the precipitation of chromium and iron nitrides in austenitic steels. This phenomenon is unfavorable because it leads to a decrease in their corrosion resistance. Only by lowering the nitriding temperature below 500 °C it is possible to carry out nitriding processes without deteriorating the corrosion resistance of nitrided steels and even improves its corrosion resistance at short process times. This is due to the formation of a hard and abrasion-resistant nitrogen-saturated austenite phase at this temperature. The duration of the nitriding process is up to 10 h for ionic methods (for other nitriding methods, it is selected depending on the type of process).
One of the types of this technology is, among others, active screen plasma nitriding (ASPN), which is becoming more and more popular among surface hardening techniques. In this case, the sample is electrically isolated from the voltage source and the plasma is formed on a screen placed around the sample [21]. In this case, the sample is heated by radiation, and ASPN generates the transfer of nitrogen mass to its surface [22, 23]. The hardness of the nitrided layer is more uniform than in the case of direct ion nitriding [24, 25], the surface roughness is lower [26], and the thickness of the layer can also be higher (with increasing temperature) [21, 27]. Many studies have shown that the efficiency of the process is influenced by such factors as geometry [28] and composition [29] of nitrided substrate material, size and surface area to material mass ratio [30], distance between the sample and the active screen (percentage of open area under the screen) [29], screen diameter [31], reactive atmosphere pressure [32], gas composition (chemical composition of the working atmosphere) [33], screen configuration [34], sample temperature [35], and nitriding process time [36].
The results presented by some authors indicate that the highest values of layer thickness and hardness were found at 80% of the N2, while in the atmosphere of pure nitrogen these values were clearly lower [33].
Using an active screen, the microscopic surface roughness was found to increase with the hole size of the screen [29] and the higher hardness resulting from the more intense movement of iron nitrides [37]. At the same time, it is stressed that reducing the diameter of the cathodic cage (and increasing the current density) results in a more reactive plasma which increases the hardness and improves corrosion resistance [38] (results of some studies indicate that there is the optimal diameter of the active screen to maximize ion flux) [31].
The distance between the sample and the screen is a critical parameter because its reduction causes a higher surface temperature of the sample [29] and an increase in the energy of nitrogen particles (at a given polarization and working pressure) affecting the increase in hardness after nitriding [39] and the thickness of the nitrided layer [34] (nitrogen diffusion towards the core and the nitrogen content in the diffusion layer increases as the distance between the screen and the sample decreases) [29].
This paper presents the results of investigations of the ionic nitriding process of 316L austenitic steel. Two variants of the position of the nitrided elements in the glow chamber of the ion nitriding apparatus were adopted: directly on the cathode and on the cathode using the active screen. After the nitriding processes were carried out, the influence of the adopted process parameters on the depth of nitrogen diffusion into the nitrided substrate was analyzed. In the further part of the work, an ionizing nitriding mechanism was proposed with the active screen method explaining the significant increase in the effectiveness of the nitriding process with the use of an active screen.
The main goal of this research was to determine what affects the intensification of the nitriding process using the active screen.
2 Research material
Austenitic steel 316L was selected for the tests. Due to its chemical composition (about 17% Cr and 12% Ni), this steel is resistant to corrosion, and a 2% addition of molybdenum prevents local corrosion (pitting). For this reason, this steel is widely used in many branches of industry, among others in medicine, mechanical engineering, and aviation as well as the chemical, food, automotive, and shipbuilding industries. It is characterized by good mechanical properties and resistance to corrosion. Nevertheless, its low wear resistance in conditions of friction limits wider use of this steel. Suitable modification of the surface layer of this steel improves its tribological properties. This issue is still an interesting research problem, both for cognitive and functional reasons [14]. The chemical composition of the examined steel (Table 1) is in accordance with certificate No. MEST 451139/2007.
3 Research methodology
In selecting the conditions of the nitriding process, the criterion of intensifying the growth rate of the nitrided layer depth was taken into account, depending on the glow discharge conditions in various areas of the working chamber of the nitriding apparatus. Two variants of placing the nitrided elements in the glow discharge chamber were adopted:
-
Directly on the cathode
-
On the cathode using an active screen.
In the first case, the surface of the samples is bombarded with ions with energies resulting from the value of cathodic drop, whereas in the second case, in the surface layer of the ion-nitrided surface of the sample, strong voltage peaks appear that react with the nitrogen ions found in this area. The time of occurrence of voltage peaks favors giving nitrogen ions and other active plasma components a high-speed glow discharge with several times higher energy than in the case of cathodic drop. High-energy nitrogen ions are driven into the material, creating a highly non-equilibrated nitrogen-saturated zone in the surface layer of the nitrided substrate. According to the Fick diffusion laws in force, this promotes the diffusion of nitrogen from the surface to the core (center) of the nitrided metallic material. Due to the nitrogen concentration gradient occurring between the surface and the core (center) of the sample, diffusion does not have to take place, at least in its initial stage, at the grain boundaries, which allows the formation of nitrided layers with high homogeneity [40].
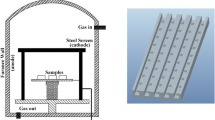
The cathode bombarded with ions is heated to the temperature enabling the nitriding process. This also causes the gas mixture surrounding the cathode to heat up and, as a result, to reduce its density, which has a major impact on the glow discharge characteristics of low-temperature plasma. This results in an increase in the mean free path of active plasma particles. Use of the active screen additionally increases the temperature of the gas mixture [14]. Diagram of the device for ion nitriding analysis is shown in Fig. 1.
The 316L austenitic steel ion nitriding process was performed in the following temperature and time ranges:
-
325–400 °C, 2–4 h (short-term and low-temperature nitriding)
-
430–490 °C, 5–17 h (long-term and high-temperature nitriding)
The chemical composition of the working atmosphere was 75% H2 + 25% N2; the pressure in the working chamber of the nitriding equipment amounted to 150 Pa.
4 Analysis of research results
For the tested austenitic steel, the following was determined:
-
The nitrogen diffusion depth—based on the analysis of the chemical composition of the elements in the surface layer (optical emission spectrometer with glow discharge HORIBA Jobin Yvon GD-Profiler HR, with Grimm discharge lamp with a cathode diameter of 4 mm).
The test of the nitrogen diffusion depth into the nitrided substrate (316L steel) as the basic feature determining the effectiveness of nitriding was used to determine the efficiency of the ion nitriding process with the active screen method. It was found that the increase in temperature, as well as prolongation of the nitriding process, resulted in an increase in the nitrogen diffusion depth in the tested material (Fig. 2), respectively:
-
316L austenitic steel (325–400 °C, 2–4 h)—increase in nitrided layer from 82 to 187% (cathode 0.56–2.51 μm, active screen 1.07–6.34 μm)
-
316L austenitic steel (430–490 °C, 5–17 h)—increase in nitrided layer from 123 to 289% (cathode 8.1–22,3 μm, active screen 18.1–72.2 μm)
5 Ionizing nitriding with active screen method
In the process of nitriding 316L austenitic steel in glow discharge conditions, the chromium nitride formed in the initial stage of the process decomposes into a lower chromium nitride and free nitrogen diffusing into the nitrided substrate. At a later stage of mass transport, the resulting lower chromium nitride decomposes into a metal atom and another portion of free nitrogen [14]:
After the nitriding process of austenitic steel, in the case of the two nitriding variations in the glow discharge plasma (I, cathode; II, cathode + active screen), the nitrided layers are made of a surface layer of CrN chromium nitride precipitates, a deeper layer of CR2N chromium nitride precipitates, and the deepest solution layer, austenite saturated with nitrogen - γN. The nitrided layer on austenitic steel is formed only on surfaces surrounded by glow discharge plasma because the chromium nitride can only form as a result of the reaction involving atomic nitrogen.
The results of the research allowed us to conclude that using the active screen in the ion nitriding process affects an increase in the depth of the nitrided layers, and this is due to [14]:
-
The higher concentration of electrons inside the active screen (Fig. 3)—this affects an increase in the concentration of active plasma components in this area, which determines the growth rate of the nitrided layer.
-
The temperature increase under active screens in relation to the cathode temperature, which in the case of nitriding austenitic steel in the atmosphere of 25% N2 + 75% H2 was in the case of the nitriding variant cathode + screen approx. 50–60 °C, depending on the set temperature of the nitriding process in the range of 400–500 °C (Fig. 4). The authors of papers [4, 41,42,43] stated that the intensity of the nitriding process depends on their chemical composition, as the glow nitriding of austenitic steel at a temperature above 500 °C simultaneously reduces the concentration of chromium, significantly worsening the corrosion resistance. Therefore, the process of glow nitriding of austenitic steel is carried out at a temperature below 500 °C.
Fig. 3 Diagram of phenomena occurring during the iron nitriding by active screen method process. Source: study based on [44]
As the temperature rises, at constant pressure, there is an increase in the free path between successive collisions of the plasma components, thus giving them more kinetic energy. The plasma particles colliding with greater kinetic energy increase the likelihood of ionization and allow the more ionized gas to carry more electric charges from the power source. It can therefore be concluded that at a higher temperature, cathodic sputtering of the surface is more effective.
The local increase in temperature under the active screen does not give a full answer to the intensification of the nitriding process using the active screen. To further explain the effect of the screen on the surrounding plasma nitrided material, voltage course tests were carried out in various areas of the glow discharge chamber (measurement diagram in Fig. 5).
Neither the comparison of the voltage course between the anode and the sample surface during the nitriding variants on the cathode and with the active screen (Fig. 6) nor their Fourier spectra (Fig. 7) showed differences on the recorded oscilloscope curves. This is due to the galvanic contact of the entire surface of the cathode with the samples and the active screen. Pulse width modulation (PWM) of a 100-Hz carrier signal resulting from the regulation of the power supplied to the power system can be observed on the oscillograms. Significant differences in the voltage course between the variant nitriding on the cathode and using the active screen were found only during their registration from the cathode fall area (measurement about 1 mm above the surface of the sample), i.e., in the zone where the potential difference in the entire glow discharge area is the largest, and accelerated by the difference in potentials, the ions and electrons have the highest kinetic energy. For the temperature range of 300–550 °C and the other adopted nitriding parameters, the width of the cathode fall (so-called cathode dark) is 1.20–1.79 mm. Significant voltage peaks were observed in the nitriding variant using the active screen. These peaks arise when the signal modulating the carrier voltage is triggered, and their occurrence is probably related to the accumulation of electric charges under the active screen at a time when the carrier voltage is gradually reduced and then quickly flows.
Knowing that the work done to transfer charge between two points in an electric field is proportional to the potential difference [45]:
where:
- WAB:
-
is the work needed to transfer the load between points A and B, J.
- UA(B):
-
is the electrical potential at point A (B), V.
- q:
-
is the electric charge, C.
Based on the received oscillograms, the effective voltage between individual measuring points was determined [46]:
where:
- USK:
-
is the effective voltage, V.
- u:
-
is the instantaneous voltage, V,
- T:
-
is the interval, s.
Analysis of the results showed that the voltage values between the surface of the shielded and unshielded sample and the anode are equal. Slight differences in values result from the fact that the compared voltage waveforms were not recorded at the same time, and the power of the current supplied by the power supply during the process is subject to continuous, small oscillations around the set value. Analysis of the voltage values between the anode and surface areas for different nitriding variants showed a significant increase in voltage, and thus the work performed by the system during the nitriding process using an active screen compared with the cathodic nitriding variant.
The occurrence of these voltage peaks in the area of cathode drop is conducive to imparting the ions high energies. In the vicinity of the surface layer, there is a current with a high intensity of voltage pulses, interacting in this area with active plasma components—ions and atoms in various excitation states. The duration of impulses with a high voltage value is conducive to ions achieving high velocity, corresponding to the value of their kinetic energy to the order of several dozen or even several hundred electronvolts. Accelerated by a large difference in potentials, the nitrogen ions are driven into the nitrided material (shallow implantation) resulting in a strong nitrogen supersaturation of the surface area. As the authors of paper [47] indicate, shallow ion implantation accompanying ion bombardment is one of the mechanisms of nitrogen transport.
The occurrence of a large concentration gradient between the near-surface zone and the deeper areas of the nitrided layer forces the diffusion of nitrogen into the nitrided surface. In addition, high-energy ions interact with the substrate, generating a large number of vacancies and increasing the specific surface area of the nitrided workpiece, which increases the intensity of nitrogen adsorption on its surface and then facilitates its absorption into the nitrided substrate. Diffusion, due to the high values of the nitrogen concentration gradient, at least at its initial stage, does not proceed only along the grain boundaries. This promotes the formation of nitrided layers of high homogeneity. A similar phenomenon also occurs, without an active screen, in the zone of strong cathode drop. However, in this case, the concentration of ions is much smaller due to the small width of this zone.
The assumed research hypothesis—the dominant significance of changes in voltage (voltage course) in the near surface layer of the nitrided 316L steel—was confirmed by the obtained and presented results.
Figure 8 shows the model of nitriding austenitic steel in nitrogen-hydrogen plasma in various glow discharge areas of direct current.
In addition to the previously discussed influence of the use of supporting screens on the change of nitriding kinetics, the use of screens makes it easier to transport the mass of nitrogen from the nitriding atmosphere because in addition to the classic model of ion nitriding in which the metal atoms are dislodged and then sputtered in the form of nitrides, additionally, dislodging of metal atoms from the surface of the screens occurs which by joining with nitrogen settle on the nitrided surface in the form of nitrides.
Confirmation of this thesis is the Armco iron profile analysis (Fig. 9), which showed that as a result of ion bombardment, sputtering of the screen surface made of 316L austenitic steel takes place, then the atoms included in the composition of the support screen are re-sputtered onto the surface of the samples in the form of free atoms or in forms of nitrogen phases (nitrides of chromium and iron). On samples made of Armco cathodic nitrided iron, chromium atoms were also found on the surface but in a much smaller amount than during nitriding with the support screen, and this was due to the fact that the furnace charge was placed on a table (cathode) made of chrome steel and with small sample sizes, chromium was sputtered as a result of dislodging atoms from the table surface (cathode).
Ionizing 316L steels using an active screen is a multi-stage process consisting of the following stages (Fig. 10):
-
Cathodic sputtering of metal atoms from the surface of both the substrate and the screen into the plasma area
-
Reactions arising during the sputtering of free metal atoms with active nitrogen atoms, which results in the formation of the CrN nitrides of these metals
-
Redeposition—deposition of CrN nitrides on the substrate surface, the authors of the paper [48] assume that cathodic sputtering and subsequent redeposition of particles play a key role in nitriding using an active screen. At the same time, they emphasize that other phenomena occurring in this process cannot be underestimated. The authors of this work stated that the introduction of an active screen proved to be a success, especially in the process of nitriding in plasma potential, where cathodic sputtering does not play a major role.
-
Decomposition of CrN nitrides, deposited on the substrate—creation of Cr2N nitrides with simultaneous formation of free nitrogen atoms
-
Diffusion of free nitrogen atoms into the substrate material
-
Desorption of excess free nitrogen atoms into the plasma area—their ionization and further participation in the nitriding process
At the same time, free nitrogen ions in the plasma, characterized by high kinetic energy, are continuously implanted into the crystal lattice of the substrate and diffuse into the substrate material. The high concentration of active plasma components under the active screen causes a simultaneous course and overlap of the presented phenomena during the nitriding process. An important role is played by the concentration of ions with high kinetic energy, which are implanted into the surface of the nitrided substrate. A nitrogen-free non-equilibrium zone is created, increasing the diffusion into the substrate material.
6 Conclusions
In the literature, issues related to ion nitriding using the active screen method are described in the context of various materials and process conditions. The authors describe in them, among others:
-
Influence of N2-H2 gas composition on the structure and properties of expanded austenite of 316L austenitic steel [49]
-
The influence of ion nitriding on the improvement of various properties of austenitic surfaces of stainless steels, such as wear resistance, electrical conductivity, and corrosion resistance [50]
-
Nitriding of stainless steels at low temperatures [51]
-
Assessment of the impact of the ion nitriding the mechanical properties of austenitic steels [36]
-
Impact of the switching power supply parameters on active plasma nitriding of the screen [52]
-
Use of the active screen method for austenitic 316 stainless steel in both nitrogen and niobium processes for the application of bipolar plates in proton exchange membrane fuel cells [53]
The research presented in this article has shown that:
-
(1)
Obtaining greater ion energy, as a result of introducing the active screen, is a decisive factor intensifying the glow discharge plasma in the nitriding processes of 316L austenitic steel.
-
(2)
Introducing the active screen causes changes in the quantitative and qualitative characteristics of the voltage. The voltage value increases, leading to increased ion density as well as their velocity—they acquire kinetic energy of approx. 250 eV. High-energy ions are implanted into the substrate material creating in the surface layer a strong non-equilibrium zone saturated with nitrogen, which in turn favors the diffusion of nitrogen into the nitrided material.
-
(3)
The use of an active screen leads to an increase in the plasma temperature affecting the substrate of metallic materials—which simultaneously reduces its density and increases the average free path of electrons and, as a result, increases the energy of ions bombarding the surface.
-
(4)
Ion bombardment and cathode sputtering affect the phase composition and the growth rate of diffused nitrided layers—during nitriding at the cathode and using the active screen, they form CrN and Cr2N nitrides in the surface layer.
-
(5)
Higher values of ion energy interacting with the substrate surface and the increase in the process temperature, as a result of introducing the active screen, intensify the physical phenomena occurring in the glow discharge plasma, which results in the formation of nitrided layers of a greater depth.
References
Hryniewicz T, Rokicki R, Rokosz K (2008) Surface characterization of AISI 316L biomaterials obtained by electropolishing in a magnetic field. Surf Coat Technol 202:1668–1673. https://doi.org/10.1016/j.surfcoat.2007.07.067
Wang X, Lei MK, Zhang JS (2007) Surface modification of 316L stainless steel with high-intensity pulsed ion beams. Surf Coat Technol 201:5884–5890. https://doi.org/10.1016/j.surfcoat.2006.10.040
Wierzchoń T (2003) Surface engineering of titanium alloys: new prospective application. Mater Sci Forum 426–432:2563–2568
Kochmański P, Nowacki J (2006) Activated gas nitriding of 17-4 PH stainless steel. Surf Coat Technol 200:6558–6562. https://doi.org/10.1016/j.surfcoat.2005.11.034
Kardonina NI, Yurovskikh AS, Kolpakov AS (2010) Transformations in the Fe – N system. Met Sci Heat Treat 52:457–467
Yurovskikh AS, Kardonina NI, Kolpakov AS (2015) Phase transformations in nitrided iron powders. Met Sci Heat Treat 57:507–514. https://doi.org/10.1007/s11041-015-9913-3
Somers MAJ (2011) IFHTSE global 21: Heat treatment and surface engineering in the twenty-first century: part 14 - development of compound layer during nitriding and nitrocarburising; current understanding and future challenges. Int Heat Treat Surf Eng 5:7–16. https://doi.org/10.1179/174951411X12956207253429
Fratczak EZ, Prieto JE, Moneta ME (2014) Growth and characterization of epitaxial iron-nitride thin films. J Alloys Compd 586:375–379. https://doi.org/10.1016/j.jallcom.2013.09.200
Borsa DM (2004) Nitride-based insulating and magnetic thin films and multilayers. Broerstraat
Tayal A, Gupta M, Kumar D, Stahn J et al (2014) Correlation between iron self-diffusion and thermal stability in doped iron nitride thin films. J Appl Phys 116:222–226
Yamashita S, Masubuchi Y, Nakazawa Y, Okayama T, Tsuchiya M, Kikkawa S (2012) Crystal structure and magnetic properties of α′′-Fe 16N 2 containing residual α-Fe prepared by low-temperature ammonia nitridation. J Solid State Chem 194:76–79. https://doi.org/10.1016/j.jssc.2012.07.025
Christiansen T, Somers MAJ (2006) Characterisation of low temperature surface hardened stainless steel. Struers J Mater 9:1–17
Baranowska J (2007) Niskotemperaturowe azotowanie stali austenitycznej. Wydawnictwo Uczelniane Politechniki Szczecinskiej, Szczecin
Frączek T (2011) Niekonwencjonalne niskotemperaturowe azotowanie jarzeniowe materiałów metalicznych. Wydawnictwo WIPMiFS Politechniki Czestochowskiej, Częstochowa
Burakowski T, Wierzchoń T (1995) Inżynieria powierzchni metali. Wydawnictwo Politechniki Warszawskiej, Warszawa
Nosei L, Farina S, Ávalos M, Náchez L, Gómez BJ, Feugeas J (2008) Corrosion behavior of ion nitrided AISI 316L stainless steel. Thin Solid Films 516:1044–1050. https://doi.org/10.1016/j.tsf.2007.08.072
Tian R, Sun J, Wang J (2008) Study on behavior of plasma nitrided 316L in PEMFC working conditions. Int J Hydrog Energy 33:7507–7512. https://doi.org/10.1016/j.ijhydene.2008.09.080
Gil L, Brühl S, Jiménez L, Leon O, Guevara R, Staia MH (2006) Corrosion performance of the plasma nitrided 316L stainless steel. Surf Coat Technol 201:4424–4429. https://doi.org/10.1016/j.surfcoat.2006.08.081
Bacci T, Borgioli F, Galvanetto E, Pradelli G (2001) Glow-discharge nitriding of sintered stainless steels. Surf Coat Technol 139:251–256. https://doi.org/10.1016/S0257-8972(01)01010-6
Skolek-Stefaniszyn E, Kaminski J, Sobczak J, Wierzchon T (2010) Modifying the properties of AISI 316L steel by glow discharge assisted low-temperature nitriding and oxynitriding. Vacuum 85:164–169. https://doi.org/10.1016/j.vacuum.2010.05.006
Ahangarani S, Mahboubi F, Sabour AR (2006) Effects of various nitriding parameters on active screen plasma nitriding behavior of a low-alloy steel. Vacuum 80:1032–1037. https://doi.org/10.1016/j.vacuum.2006.01.013
Andrea SB, Kocsisné Baán M, Marosné Berkes M (2012) Nitridálás – korszerű eljárások és vizsgálati módszerek. Miskolci E, Miskolc
Li CX, Georges J, Li XY (2002) Active screen plasma nitriding of austenitic stainless steel. Surf Eng 18:453–457. https://doi.org/10.1179/026708402225006240
Han L, Dai JT, Huang XR, Zhao C (2013) Study on the fast nitriding process of active screen plasma nitriding. Phys Procedia 50:94–102. https://doi.org/10.1016/j.phpro.2013.11.017
Zhao C, Li CX, Dong H, Bell T (2006) Study on the active screen plasma nitriding and its nitriding mechanism. Surf Coat Technol 201:2320–2325. https://doi.org/10.1016/j.surfcoat.2006.03.045
Corengia P, Ybarra G, Moina C, Cabo A, Broitman E (2005) Microstructural and topographical studies of DC-pulsed plasma nitrided AISI 4140 low-alloy steel. Surf Coat Technol 200:2391–2397. https://doi.org/10.1016/j.surfcoat.2005.01.060
Naeem M, Zaka-ul-Islam M, Shafiq M, Bashir MI, Díaz-Guillén JC, Zakaullah M (2017) Influence of cathodic cage diameter on mechanical properties of plasma nitrided AISI 304 steel. Surf Coat Technol 309:738–748. https://doi.org/10.1016/j.surfcoat.2016.10.093
Figueroa CA, Alvarez F (2005) New pathways in plasma nitriding of metal alloys. Surf Coat Technol 200:498–501. https://doi.org/10.1016/j.surfcoat.2005.02.089
Nishimoto A, Nagatsuka K, Narita R, Nii H, Akamatsu K (2010) Effect of the distance between screen and sample on active screen plasma nitriding properties. Surf Coat Technol 205:S365–S368. https://doi.org/10.1016/j.surfcoat.2010.08.034
Alves C, Da Silva EF, Martinelli AE (2001) Effect of workpiece geometry on the uniformity of nitrided layers. Surf Coat Technol 139:1–5. https://doi.org/10.1016/S0257-8972(00)01146-4
Naeem M, Shafiq M, Zaka-ul-Islam M, Ashiq A, Díaz-Guillén JC, Shahzad M, Zakaullah M (2016) Enhanced surface properties of plain carbon steel using plasma nitriding with austenitic steel cathodic cage. Mater Des 108:745–753. https://doi.org/10.1016/j.matdes.2016.07.044
Wang S, Cai W, Li J, Wei W, Hu J (2013) A novel rapid D.C. plasma nitriding at low gas pressure for 304 austenitic stainless steel. Mater Lett 105:47–49. https://doi.org/10.1016/j.matlet.2013.04.031
De Sousa RRM, De Araújo FO, Gontijo LC et al (2014) Cathodic cage plasma nitriding of austenitic stainless steel (AISI 316): influence of the working pressure on the nitrided layers properties. Mater Res 17:427–433. https://doi.org/10.1590/S1516-14392013005000197
Nishimoto A, Matsukawa T, Nii H (2014) Effect of screen open area on active screen plasma nitriding of austenitic stainless steel. ISIJ Int 54:916–919. https://doi.org/10.2355/isijinternational.54.916
Yazici M, Çomakli O, Yetim T et al (2015) The effect of plasma nitriding temperature on the electrochemical and semiconducting properties of thin passive films formed on 316L stainless steel implant material in SBF solution. Surf Coat Technol 261:181–188. https://doi.org/10.1016/j.surfcoat.2014.11.037
Hoshiyama Y, Mizobata R, Miyake H (2016) Mechanical properties of austenitic stainless steel treated by active screen plasma nitriding. Surf Coat Technol 307:1041–1044. https://doi.org/10.1016/j.surfcoat.2016.07.032
Taherkhani F, Taherkhani A (2010) Surface characterization of through cage plasma nitriding on the surface properties of low alloy steel. Sci Iran 17:253–263
Naeem M, Shafiq M, Zaka-ul-Islam M, Nawaz N, Díaz-Guillén JC, Zakaullah M (2016) Effect of cathodic cage size on plasma nitriding of AISI 304 steel. Mater Lett 181:78–81. https://doi.org/10.1016/j.matlet.2016.05.144
Hubbard P, Dowey SJ, Partridge JG, Doyle ED, McCulloch DG (2010) Investigation of nitrogen mass transfer within an industrial plasma nitriding system II: application of a biased screen. Surf Coat Technol 204:1151–1157. https://doi.org/10.1016/j.surfcoat.2009.08.030
Frączek T, Ogórek M, Skuza Z (2020) The effectiveness of active screen method in ion nitriding grade 5 Eli Titanium alloy. Metalurgija 59:167–170
Frączek T, Olejnik M, Skuza Z, Kolmasiak C (2009) Efektywność azotowania jarzeniowego stali austenitycznej X2CrNiMo17-12-2. In: Mróz J (ed) Produkcja i zarządzanie w hutnictwie. Wyd.WIPMiFS PCzęst, pp 102–105
Sobiecki JR, Kazior J, Wierzchoń T (2005) Niskotemperaturowe azotowanie jarzeniowe spiekanej stali austenitycznej. Inżynieria Mater 26:434–436
Jiang LG, Peng Q, Li C et al (2008) Effect of DC plasma nitriding temperature on microstructure and dry-sliding wear properties of 316L stainless steel. Surf Coat Technol 202:2749–2754. https://doi.org/10.1016/j.surfcoat.2007.10.002
Ahangarani S, Sabour AR, Mahboubi F, Shahrabi T (2009) The influence of active screen plasma nitriding parameters on corrosion behavior of a low-alloy steel. J Alloys Compd 484:222–229. https://doi.org/10.1016/j.jallcom.2009.03.161
Bolkowski S (2018) Elektrotechnika. WSiP, Warszawa
Olejnik M (2011) Niskotemperaturowe i krótkookresowe azotowanie jarzeniowe stali austenitycznej X2CrNiMo17-12-2. Politechnika Częstochowska
Sokolowska A, Rudnicki J, Beer P, Maldzinski L, Tacikowski J, Baszkiewicz J (2001) Nitrogen transport mechanisms in low temperature ion nitriding. Surf Coat Technol 142–144:1040–1045. https://doi.org/10.1016/S0257-8972(01)01260-9
Corujeira Gallo S, Dong H (2009) On the fundamental mechanisms of active screen plasma nitriding. Vacuum 84:321–325. https://doi.org/10.1016/j.vacuum.2009.07.002
Dalke A, Burlacov I, Hamann S, Puth A, Böcker J, Spies HJ, Röpcke J, Biermann H (2019) Solid carbon active screen plasma nitrocarburizing of AISI 316L stainless steel: influence of N2-H2 gas composition on structure and properties of expanded austenite. Surf Coat Technol 357:1060–1068. https://doi.org/10.1016/j.surfcoat.2018.10.095
Lin K, Li X, Dong H, Guo P, Gu D (2018) Nitrogen mass transfer and surface layer formation during the active screen plasma nitriding of austenitic stainless steels. Vacuum 148:224–229. https://doi.org/10.1016/j.vacuum.2017.11.022
Pinedo CE, Larrotta SIV, Nishikawa AS, Dong H, Li XY, Magnabosco R, Tschiptschin AP (2016) Low temperature active screen plasma nitriding of 17–4 PH stainless steel. Surf Coat Technol 308:189–194. https://doi.org/10.1016/j.surfcoat.2016.07.096
Naeem M, Waqas M, Jan I, Zaka-ul-Islam M, Díaz-Guillén JC, Rehman NU, Shafiq M, Zakaullah M (2016) Influence of pulsed power supply parameters on active screen plasma nitriding. Surf Coat Technol 300:67–77. https://doi.org/10.1016/j.surfcoat.2016.05.032
Lin K, Li X, Tian L, Dong H (2015) Active screen plasma surface co-alloying of 316 austenitic stainless steel with both nitrogen and niobium for the application of bipolar plates in proton exchange membrane fuel cells. Int J Hydrog Energy 40:10281–10292. https://doi.org/10.1016/j.ijhydene.2015.06.010
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fraczek, T., Ogorek, M., Skuza, Z. et al. Mechanism of ion nitriding of 316L austenitic steel by active screen method in a hydrogen-nitrogen atmosphere. Int J Adv Manuf Technol 109, 1357–1368 (2020). https://doi.org/10.1007/s00170-020-05726-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00170-020-05726-8