Abstract
Purpose
Recent studies have shown that the incidence of glove lesions during arthroscopy is much lower than that during primary and revision arthroplasty. However, the rate of glove damage after knot tying has not yet been systematically recorded. Therefore, the aim of the study was to determine the impact of surgical knot tying on glove integrity. It was hypothesized that knot tying increases the rate of glove damage, especially in arthroscopic surgery, which could be of special relevance in the treatment of rotator cuff tears.
Methods
Gloves that were changed immediately before suturing and only worn during knot tying were investigated for their integrity by means of water tightening test according to EN455. A total of 234 gloves from 40 total hip arthroplasties (THAs), 42 total knee arthroplasties (TKAs) and 36 rotator cuff repairs (RCRs) were collected. A bacterial pass-through test (BPTT) on glove lesions was performed under simulated sterile surgical conditions for 3 surgeons after a wear duration of 45 min.
Results
Glove damage by knot tying occurred in 25% of THA, 36.6% of TKA and 25% of RCR surgeries. In THA, the pulling hand (PH) was affected in 46.2%, and the main area of damage (15.4%) was detected on the tip of the middle finger; in TKAs the PH was damaged in 75%, and in RCRs the PH was affected in 66.7%, with most of the lesions (20% each) occurring on the tip of the index finger and the ring finger. The BPTT showed Staphylococcus hominis and Bacillus cereus.
Conclusion
Intraoperative knot tying causes damage to gloves, which is of special relevance for arthroscopic surgery. Whereas knot tying is only partly responsible for glove damage in arthroplasty, the general rate of glove damage in arthroscopic surgery is low without knot tying. The surgical knot tying process must be understood as a possible damaging impact on the glove. Therefore, single gloving is not recommended, which is especially important in arthroscopic surgery, where double gloving is not yet standard.
Level of Evidence
IV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevention of infections during surgical procedures is of great importance, with the hand of the surgeon being one possible source of pathogens. In addition to surgical hand disinfection, the surgical glove is one of the most important cornerstones in the prevention of infections for the patient and the surgical team [16]. Recent studies have shown high rates of glove damage in joint and revision arthroplasty, procedures where gloves are subjected to repetitive mechanical stress during surgery. As a result, the integrity of the gloves is compromised [4, 10, 11, 33]. However, glove damage was also described in previous studies during much less mechanically demanding operations, such as joint arthroscopies and laparoscopic procedures [9,10,11, 22].
It is conceivable that particularly larger damage to the gloves can occur in major orthopaedic surgeries, such as hip and knee or revision arthroplasty, due to mechanical stress and the instruments used, whereas in low-impact operations, such as shoulder arthroscopy, the lesion rate is lower, and the majority of lesions are one millimetre or less (microlesions) in size [10, 11]. However, the causes of these lesions have not yet been clarified. Whether procedures such as tying fascia during hip or knee arthroplasty or the technique in the treatment of rotator cuff lesions have any effect has not yet been systematically investigated in the literature. The influence of knot tying has mainly been described under laboratory settings [14, 23]. Intraoperative studies under real surgical conditions are rare; in particular, the influence of intraoperative surgical knot tying on sterile gloves in minimally invasive shoulder surgery is poorly understood [20]. To our knowledge, this is the first study to measure the extent of damage to gloves by intraoperative knot tying.
As surgical gloves are inexpensive single-use items, their importance is commonly underestimated. Many surgeons rely on standardized regulations (EN 455, ASTM D3577) regarding infection and self-protection. It goes largely unnoticed that they are production rather than protection standards. Intraoperative mechanical stress is not represented in any of these standards. EN 455 defines holes as a defect in the glove that allows water to leak out. For the water tightening test, a glove is clamped in a holding device and filled with 1000 ml of water (temperature = 15–35 °C); if no water leaks out within 2–3 min, the glove is considered leak-proof. A production lot must meet a leak tightness level of an acceptable quality limit (AQL) of 0.65. Mechanistically, only the tensile force of the entire glove is tested, which must be above 9 newtons (1 N = weight of a bar of chocolate) [13].
A coherent study of the influence of knot tying on glove damage in the three major joints (shoulder, hip, knee) has not yet been available in the literature. In particular, differences in the localization and size of glove damage among the entities have not been analysed. Differences in damage configuration between penetrating and friction lesions have not been documented thus far. For surgeons, knowledge of the different susceptibilities to glove damage depending on the joint is extremely important for preventing complications. However, glove lesions could play an important role in both self-protection and patient safety. The hypothesis is that the damage due to knot tying in the shoulder, hip and knee interventions differs in localization and size depending on the type of surgery.
Materials and methods
At the orthopaedic clinic and policlinic, gloves from 40 total hip arthroplasty (THA), 41 total knee arthroplasty (TKA) and 36 arthroscopic rotator cuff repairs (RCR) were collected from March until August 2020. From these interventions, 234 gloves were subsequently tested with water tightening test according to the European standard EN 455. Ethics approval for the study was granted by the local ethics committee (A2016-0112), and data protection requirements were observed.
The suture material for the closure of the fascia/capsule after hip and knee joint replacement was Vicryl 2 (Ethicon, Inc., Johnson & Johnson Intl., NJ, USA). The sutures on the rotator cuff were stitched with Fibrewire 2 (Arthrex, Inc., FL, USA). THA and TKA were sutured with two concurrent knots presented as slip knots, the fascia was closed in tension, and an opposing knot was tied under tension conditions. This was followed up by two further safety knots. In RCR, seven knots were tied arthroscopically as a standard of secure treatment. The pulling thread was passed through a knot pusher, and the end of the thread was looped several times around the ring finger of the pulling hand. Two identical knots were tied with the looping hand as slip knots and pushed to the end of the thread with the knot pusher, followed by a reverse locking knot. Further safety knots were tied in 2 to 1 alternations for a total 7 knots.
The gloves of the knot-tying surgeons were collected and packed at the end of each surgery, and the data relevant for evaluation were documented. The total number of gloves, number of gloves per operation, and type and duration of the operation were recorded. A separate pair of gloves was worn solely for the knot tying procedure (tendon, capsule, and fascia) to exclude damage from the previous steps of the surgery. All surgeries were performed with sterile, powder-free latex gloves for single use (ProtexisTM, Cardinal Health Dublin, Ohio, USA (AQL 0.65). The examination of the gloves was conducted at the Biomechanics and Implant Technology Research Laboratory of the Orthopaedic Clinic and Policlinic. To determine the baseline of holes during production, 100 unused gloves from the manufacturer were tested. As a control, the influence of glove undressing was tested on 50 gloves without surgery. The evaluation was performed according to standard EN 455, “Medical gloves for single use—Part 1”, a method for testing for freedom from holes with the water tightening test (Fig. 1). The localization of the damage was determined, the size and dimension were measured with a plastic goniometer (Kirchner & Wilhelm GmbH & Co. KG, Asperg, Germany) with an accuracy of ± 1 mm, and the lesion configuration was recorded microscopically (Digital Microscope VHX-6000, Keyence, Germany).
Illustration of glove lesions during the water tigtening test in ascending size. Figure A shows the typical drop formation of a microlesion (< 1 mm). Figure B shows the water leakage from a 2 mm lesion (left finger shown) and a 4 mm lesion (right finger shown). The test fluid was stained with blue ink for illustrative purposes
To analyse the possible passage of bacteria through a glove lesion, simulated surgical conditions without a patient were set up. Three surgeons, who were dressed in a sterile gown and one pair of sterile gloves, disinfected their hands with Sterillium (PAUL HARTMANN AG, Heidenheim, Germany) for 3 min according to EN 12,791 [12]. This was followed by the handling of surgical instruments (hammer, clamps, scissors, luers, etc.) in the operating room under lamina air flow conditions for 45 min. After 22 min into the wear duration (45 min), the fingertips of the gloves were intentionally damaged palmar with a solid surgical spring-eyed needle (HS54, OESHS54, RESORBA Medical GmbH, Nuremberg, Germany). Each fingertip was punctured with a separate sterile needle. Instrument use was performed for an additional 13 min, followed by 10 min of free manual and arthroscopic knot tying with knot sliding instruments. Subsequently, each finger was pressed onto an aerobic and anaerobic agar plate (COLUMBIA 5% SB and SCHAEDLER 5% SB, Becton Dickinson GmbH, Heidelberg, Germany) for 4 s under sterile conditions. The plates were microbiologically examined in the in-house Institute for Microbiology, Virology and Hygiene according to German microbiological standards at a microbiological laboratory accredited by the national accreditation organisation of the Federal Republic of Germany (DAkkS) DIN EN ISO 15189 and DIN EN ISO/IEC 17,025. A total of 120 samples were taken and evaluated. Among them, 60 samples were in the “glove lesion” group, with 30 aerobic and 30 anaerobic samples. In the “gloveless” group, 60 samples were taken, including 30 aerobic and 30 anaerobic samples. The agar plates were incubated for a total of 5 days. As a control, each surgeon performed the same procedure under the same setup without gloves the next day (comparison group).
Statistical analysis
The collected data were analysed using SPSS Statistics Package Version 22 (IBM Corp., New York, USA). Descriptive statistics were calculated for continuous and categorical variables. Continuous variables are displayed as the mean values and standard deviations (SD) as well as the median and range, as most of the data were not normally distributed. Categorical factors are shown as frequencies (n) with percentages in brackets. Testing for differences between different types of operations of categorical factors was performed by Pearson's chi-square test. The significance level was set at p < 0.05. Sample size calculation was based on analysis with ANOVA (Cohen`s f = 0.2; alpha level 0.05; power 0.7). A total of 198 test items were calculated, with 66 test items per intervention group, resulting in 33 surgical procedures.
Results
Overview data and examined surgical treatment
In THA and TKA, knot tying procedures were performed by a total of 10 endoprosthetic surgeons, while three shoulder specialists performed knot tying in the rotator cuff interventions as well as in THA and TKA. In the arthroplasties, seven tying surgeons used the left hand to pull the thread and knotted over the thread with the right hand, and six knotters used the right hand to pull the thread. In RCR, two of the three shoulder surgeons used the knot pusher in the right hand, looping the ring finger with the pulling thread several times and then tying the right hand over the thread. One surgeon used the knot pusher in the left hand, but the knot tying technique itself was identical. All surgeons were right-handed.
Glove handling
In the determination of the baseline, no prior damage to the 100 tested gloves was detected. The glove batch was within the AQL of 0.65. Of the 50 gloves that were put on and taken off, none showed damage.
Number of glove lesions, localization on the glove and size
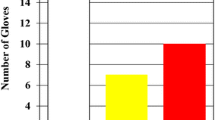
In total, 234 gloves were collected, and 48 instances of damage were detected. Detailed information is provided in Tables 1 and 2, as well as in Fig. 2. Penetrating and friction injuries showed different injury patterns. Figure 3 shows a typical knot tying and penetrating lesion.
Images A and B show typical friction lesions with tear formation in the glove after the use of nonabsorbable suture material for suturing tendons (A in 20 × magnification, B in 200 × magnification). C and D show the Fiberwire suture material used (C in 20 × magnification, B n 200 × magnification). E and F show a hole caused by a 20G cannula (E at 20 × magnification, F at 200 × magnification). G and H show a typical cannula used during arthroscopy (20G 0.9 × 2 3/4)
Bacterial pass-through testing
Evaluations occurred on Days 1, 2 and 5 (anaerobic only on Day 5). In the samples of the "glove lesion" group, S. hominis was detected for Surgeon 2 on the left index finger (1 colony-forming unit (CFU) anaerobic) and Surgeon 3 on the right small finger (1 aerobic CFU and 1 anaerobic CFU). In the "gloveless" group, B. cereus was detected for Surgeon 1 (thumb: 6 aerobic CFUs and 9 anaerobic CFUs, index finger: 1 aerobic CFUs/3 anaerobic CFUs, middle finger: 8 aerobic CFUs/5 anaerobic CFUs, ring finger: 10 aerobic CFUs/7 anaerobic CFUs, little finger: 8 aerobic CFUs/5 anaerobic CFUs) and Surgeon 2 (index finger: 1 anaerobic CFU, ring finger: 1 anaerobic CFU). In the anaerobic approach, no obligate anaerobes were found.
Discussion
The most important findings of our study were as follows: (1) Knot tying is a potential cause of glove damage in RCRs, THAs, and TKAs. (2) The localization of glove damage varies between joints and types of intervention. (3) Lesion size was found to be predominantly less than 1 mm (microlesions). (4) Microlesions allow bacterial passage, thus providing a potential intraoperative source of contamination, especially in single gloving.
To date, there is limited information on the exact origin of the damage and when it occurs in the time course of an intervention [26].
The development of microlesions and the influence of surgical knots on glove microlesions have not been conclusively clarified [14, 23]. The process of knot tying appears to permanently damage the integrity of the glove. As shown in this study, surgical knot tying primarily caused microlesions. Thus, some of the lesions on gloves described in the literature could be explained, even during mechanically less demanding procedures such as soft tissue surgery [9, 24, 32]. As shown, surgical hand disinfection can provide an effect of germ reduction over a period of 45 min, but longer-duration surgeries could result in recurrence of pathogen formation, as alcohol-based disinfectants have a rapid germicidal effect on the skin but no lasting residual activity [27, 28]. As the recovery of bacterial skin flora after the use of hand disinfection is slow, glove lesions could play a role in prolonged operations or in the later course of the surgery. [15].
Because the knot tying process occurs mainly at the end of an operation, microlesions could play a special role, as skin flora forms again in the course of time; thus, the risk of contamination of the sterile joint cavities could occur. The size of the lesion is only of minor importance here because pathogens with a size of less than 1.0 µm are considerably smaller than the damages found and can thus penetrate through them easily. The friction and traction force created by the suture at the interface between skin and glove and suture and glove as well as other forces could therefore lead to contamination if this occurs in a lesion area [23]. In particular, the highest damage rate on the pulling index finger was found across different clinical disciplines and types of surgery, and this could play a role in surgical site infections in this context.
While double gloving was performed in this study, double gloving should be a widely used standard, and it is not consistently used in other clinical disciplines [3, 21]. In an experimental ex vivo study by Battersby et al., double gloving was associated with a reduction in knot-tying quality. Thus, which risk is more serious for the patient, a qualitatively poorer knot or the risk of infection, must be considered [2]. Balancing the risks is important, especially as the loss of the protective function of the glove was proven to allow bacteria to penetrate through these lesions [17], and even small amounts of bacteria could be sufficient to trigger an implant or suture material-associated infection [31]. Despite surgical hand disinfection, germs can pass through corresponding glove punctures, even if the germ load at less than 100 CFUs was low [29]. The detection of S. hominis—a common commensal on human skin—in the BPTT represents a very likely scenario for contamination, as it could occur during surgery. The B. cereus found on the unprotected skin of surgeons is a spore-forming bacterium that may show pathogenicity in surgery [7] but is mainly thought to be responsible for acute diarrhoea in humans [25]. The spores are often not safely eliminated by common hand disinfectants and hand washing [30]. An undamaged glove could prevent infection. However, further studies should be performed on how long hand disinfection can keep the hands sterile during orthopaedic surgery.
Of note, mechanical stress loading and its damaging effects are rarely considered in the known standards for manufacturing and testing gloves of the European Committee for Standardization (CEN) European Standard (EN) EN455 or American Society for Testing and Materials (ASTM D3577). To test for damage, only a random water tightening test is mandatory. During this test, the gloves must be leak-tight for 2–3 min when filled with 1000 ml of water, and higher stresses are not tested [1, 13]. Thus, randomly tested, the glove and the entire batch are considered to be leak-proof and free from damage. According to the standard, the product batch is allowed an accepted quality level (AQL) of 0.65 (EN455); for example, for a batch size of 500,000 pieces, only 315 gloves must be randomly tested, among which 5 pieces may show damage (AQL 0.65; 5 damages per 315 gloves) and still allow the batch to be placed on the market [1, 13]. In the test of mechanical load, the unused glove must carry a weight of 100 N for a short time, and further tests, including a test of the glove after the mechanical load, are not intended [13]. Other infection protection products, such as condoms, are subject to higher quality (AQL of 0.25; 2 instances of damage per 315 condoms) and are tested and standardised by the industry in a more complex process than surgical gloves [6].
In this context, the integrity of the glove as protection for surgical staff must also be clearly mentioned. While the transmission of bacteria primarily represents a loss of asepsis and a risk of infection for the patient, microlesions play a major role in the transmission of pathogenic viruses to the surgical team. Studies with pass-through tests of viruses can be found in the literature, but most of them are 20 years old and older [5, 34]. At that time, a latex glove was considered virus-proof if it was sufficiently vulcanised, and a liquid must exist as a transport medium (sweat and bodily fluids) [19]. Given the size of viruses in the nanometre range, however, even the smallest lesions and longer operation times could be sufficient to achieve transmission. Burn et al. showed that epithelial cell particles of the surgeon could be detected in the suture material despite wearing a glove. It shows how vulnerable gloves are and how an exchange of biomaterial can occur [3]. Therefore, microlesions could represent a major health risk for the surgical team, especially for high-risk patients with viral infections such as hepatitis or human immunodeficiency virus (HIV) [8, 18]. The skin lesions on the fingers resulting from knot tying could possibly serve as a port of entry. The results of Giordano et al. could not be substantiated by our study. Giordano et al. reported no risk of perforation of surgical gloves during knot tying and suturing and stated that the skin abrasions on fingers that occurred must have been caused by friction; thus, there was no risk of perforation of surgical gloves [14]. However, the static setup of their study may have neglected soft-tissue-related bouncing effects that occur under real surgical conditions and produce saw-like movements that could have led to the lesions on the collected gloves. In a further experimental approach, arthroscopic suture materials for rotator cuff repairs were examined for their lesion-causing characteristics; here, the potential for glove damage was certainly evident [23]. Unfortunately, the study did not report data on the size of the lesions caused by suture material. Although Kaplan et al. confirmed the damage caused by gloves during arthroscopy, there was no indication of the size of the lesions in these relevant studies. Furthermore, as experimental studies do not reflect the exact surgical environment, the influences of movements, tissue elasticity, body fluids and mechanical loads are missing [14, 20, 23].
In this context, it must be clearly emphasized that an AQL of 0.65 (EN 455) with a production-related basic rate of lesions during glove production does not provide sufficient protection against viral infections. As limitation of the study, two arthroplasties (THA and TKA) and one arthroscopic procedure (RCR) were included. However, the influence of different knot tying techniques in various anatomical structures (e.g., tendon/fascia) was investigated. The knot tying process itself is subject to the individual preferences of each surgeon. Despite specific instruction on knot tying, it was difficult to standardize the procedure due to multiple factors (e.g., strength, direction and speed of pull). However, lesions occurred in the gloves of all participating surgeons, thus suggesting that the damage is not the result of individual preferences and practices. The subsequent analysis in the test laboratory did not allow us to distinguish between lesions caused by the production process or lesions occurring during the procedure. Here, an improved analysis of the lesions, e.g., digital or electron microscopic analysis, could provide more information. As only gloves with a positive water tightening test were examined under the microscope for damage, further damage could therefore be undetected. It cannot be excluded that smaller lesions that occurred intraoperatively were increased in size when removing the glove. The effects on the postoperative wound infection rate as well as possible infections of the medical staff were not investigated.
Based on the findings, more attention should be given to the intraoperative use of gloves. The glove is subject to wear from lesions during the procedures, which is intensified or increased by tying knots. In the standards, water-retaining gloves are considered to be tight and therefore impermeable to viruses and other pathogens. Microlesions destroy the protective barrier and thus protect the patient and the surgeon. Glove-changing algorithms and double gloving should be established in the daily routine to ensure the best possible protection. The clinical benefit of the study would be to increase the awareness of the surgical teams of intraoperative damage of surgical gloves, their main damage areas in the related surgeries and corresponding mindfulness for infection prevention for the patient and surgical team. Furthermore, knot tying should be recognized as the cause of microlesions, and intraoperative glove-changing algorithms should be established.
Conclusion
The very process of surgical knot tying leads to microlesions and thus the loss of integrity of the glove. The protective function of the glove against the transmission of viral and bacterial pathogens could be lost due to the microlesions. In surgeons, the skin lesions resulting from knot tying could in turn represent a convenient port of entry for pathogens. Regular glove changes, especially after knot tying and at defined intervals, are recommended. Additionally, gloves should be subject to more stringent standards, as mechanical stress and protection of the surgical team and the patient are not sufficiently considered. A requirement that would emerge from this study could be that gloves should be optimized in design and material and tailored to surgical requirements to limit wear.
Abbreviations
- AQL:
-
Acceptable quality level
- ASTM:
-
American Society for Testing and Materials
- BPTT:
-
Bacterial pass-through test
- CEN:
-
European Committee for Standardization
- CFU:
-
Colony-forming unit
- DAkkS:
-
National accreditation organization of the Federal Republic of Germany
- DIN:
-
Deutsche Industrie Norm
- EN:
-
European Standard
- HIV:
-
Human immunodeficiency virus
- IEC:
-
International Electrotechnical Commission
- ISO:
-
International Organization for Standardization
- N:
-
Newton
- PH:
-
Pulling hand
- RCR:
-
Rotator cuff repair
- SD:
-
Standard deviation
- LH:
-
Looping hand
- THA:
-
Total hip arthroplasty
- TKA:
-
Total knee arthroplasty
References
American Society for Testing and Materials (2019) ASTM D3577−19: Standard Specification for Rubber Surgical Gloves. ASTM Int’l. www.astm.org/d3577-19.html. (accessed 08.08.2022)
Battersby CLF, Battersby NJ, Hollyman M, Hunt JA (2016) Double-Gloving Impairs the Quality of Surgical Knot Tying: A Randomised Controlled Trial. World J Surg 40:2598–2602
Burn MB, Holtorf HL, Smith KM, Bernstein DT, Delgado DA, Prudhomme N, Deavers MT, McCulloch PC, Harris JD (2017) Do Skin Lacerations Imply Tissue Transfer From Surgeon to Patient During Arthroscopic Knot Tying? Arthroscopy 33:2248–2254
Chan KY, Singh VA, Oun BH, To BHS (2006) The rate of glove perforations in orthopaedic procedures: single versus double gloving A prospective study. Med J Malaysia 61:3–7
Dalgleish AG, Malkovsky M (1988) Surgical gloves as a mechanical barrier against human immunodeficiency viruses. Br J Surg 75:171–172
Deutsches Institut für Normung (2015) EN ISO 4074:2015 - Natural rubber latex male condoms - Requirements and test methods (ISO 4074:2015). Beuth Verlag. www.beuth.de/de/norm/din-en-iso-4074/263522064. (accessed 08.08.2022)
Dubouix A, Bonnet E, Alvarez M, Bensafi H, Archambaud M, Chaminade B, Chabanon G, Marty N (2005) Bacillus cereus infections in Traumatology-Orthopaedics Department: retrospective investigation and improvement of healthcare practices. J Infect 50:22–30
Edmiston CE, Zhou SS, Hoerner P, Krikorian R, Krepel CJ, Lewis BD, Brown KR, Rossi PJ, Graham MB, Seabrook GR (2013) Evaluation of an antimicrobial surgical glove to inactivate live human immunodeficiency virus following simulated glove puncture. Surgery 153:225–233
Enz A, Kamaleddine I, Groß J, Schafmayer C, Alwafai E, Sievers L, Mittelmeier W, Klinder A (2021) Is single gloving still acceptable? investigation and evaluation of damages on sterile latex gloves in general surgery. J Clin Med 10:3887
Enz A, Klinder A, Mittelmeier H, Kundt G, Mittelmeier W, Zaatreh S (2018) Damages with high consequences: analysis of perforations in surgical latex operation gloves from orthopedic surgeries. Eur J Microbiol Immunol (Bp) 8:159–162
Enz A, Kostuj T, Warnke P, Osmanski-Zenk K, Mittelmeier W, Klinder A (2022) Intraoperative damage to surgical gloves during various operations on the musculoskeletal system: a multicenter study. Arch Orthop Trauma Surg 142:57–65
European Committee for Standardization (2018, January) Chemical disinfectants and antiseptics - Surgical hand disinfection - Test method and requirements (phase 2, step 2); German version EN 12791:2016+A1:2017. Beuth Verlag. www.beuth.de/de/norm/din-en-12791/280780004 (accessed 08.08.2022)
European Committee for Standardization (2020) CEN EN455–1:2020 - Medical gloves for single use - Part 1: Requirements and testing for freedom from holes. Beuth Verlag. www.beuth.de/de/norm/din-en-455-1/317510501 (accessed 08.08.2022)
Giordano V, Koch HA, de Sousa PJ, de Morais LS, de Araújo HR, de Souza FS, do Amaral NP (2014) Is the surgical knot tying technique associated with a risk for unnoticed glove perforation? An exp study Patient Saf Surg 8:26. https://doi.org/10.1186/1754-9493-8-26
Gold NA, Mirza TM, Avva U (2022) Alcohol Sanitizer. StatPearls StatPearls Publishing, Treasure Island (FL)
Hansis M, Kramer A, Mittelmeier TM, Exner M, Mielke M, Exner M (2018) Prevention of postoperative wound infections. recommendation of the German commission for hospital hygiene and infection prevention (KRINKO) at the Robert koch institute. - Prävention postoperativer Wundinfektionen. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz 61:448–473
Harnoss J-C, Partecke L-I, Heidecke C-D, Hübner N-O, Kramer A, Assadian O (2010) Concentration of bacteria passing through puncture holes in surgical gloves. Am J Infect Control 38:154–158
Johnson GK, Nolan T, Wuh HC, Robinson WS (1991) Efficacy of glove combinations in reducing cell culture infection after glove puncture with needles contaminated with human immunodeficiency virus type 1. Infect Control Hosp Epidemiol 12:435–438
Kamp W-D, Lenk V (1991) Investigations on the impermeability of disposable gloves to microorganisms. untersuchungen zur dichtigkeit von einmalhandschuhen gegen mikroorganismen. Hyg Med 16:287–292
Kaplan KM, Gruson KI, Gorczynksi CT, Strauss EJ, Kummer FJ, Rokito AS (2007) Glove tears during arthroscopic shoulder surgery using solid-core suture. Arthroscopy 23:51–56
Lipson ME, Deardon R, Switzer NJ, de Gara C, Ball CG, Grondin SC (2018) Practice and attitudes regarding double gloving among staff surgeons and surgical trainees. Can J Surg 61:244–250
Manjunath AP, Shepherd JH, Barton DPJ, Bridges JE, Ind TEJ (2008) Glove perforations during open surgery for gynaecological malignancies. BJOG 115:1015–1019
Martinez A, Han Y, Sardar ZM, Beckman L, Steffen T, Miller BS, Martineau PA (2013) Risk of glove perforation with arthroscopic knot tying using different surgical gloves and high-tensile strength sutures. Arthroscopy 29:1552–1558
Matsuoka S, Kondo T, Seishima R, Okabayashi K, Tsuruta M, Shigeta K, Ishida T, Hasegawa H, Kitagawa Y (2022) Surgical glove perforation during laparoscopic colorectal procedures. Surg Endosc 36:3489–3494
McDowell RH, Sands EM, Friedman H. Bacillus Cereus. 2021 Sep 16. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022 Jan. PMID: 29083665
Medhioub F, Jaber E, Hamrouni A, Gharbi L (2020) Unnoticed surgical gloves intraoperative perforation: a multicentric study of the leading factors. Jr med res 3(3):9–12. https://doi.org/10.32512/jmr.3.3.2020/9.12
Mulberrry G, Snyder AT, Heilman J, Pyrek J, Stahl J (2001) Evaluation of a waterless, scrubless chlorhexidine gluconate/ethanol surgical scrub for antimicrobial efficacy. Am J Infect Control 29:377–382
Olson LKM, Morse DJ, Duley C, Savell BK (2012) Prospective, randomized in vivo comparison of a dual-active waterless antiseptic versus two alcohol-only waterless antiseptics for surgical hand antisepsis. Am J Infect Control 40:155–159
Parienti JJ, Thibon P, Heller R, Le Roux Y, von Theobald P, Bensadoun H, Bouvet A, Lemarchand F, Le Coutour X, Bensadoun H, Antisepsie Chirurgicale des mains Study Group (2002) Hand-rubbing with an aqueous alcoholic solution vs traditional surgical hand-scrubbing and 30-day surgical site infection rates: a randomized equivalence study. JAMA 288(6):722
Sasahara T, Ae R, Watanabe M, Kimura Y, Yonekawa C, Hayashi S, Morisawa Y (2016) Contamination of healthcare workers’ hands with bacterial spores. J Infect Chemother 22:521–525
Van Wijngaerden E, Peetermans WE, Vandersmissen J, Van Lierde S, Bobbaers H, Van Eldere J (1999) Foreign body infection: a new rat model for prophylaxis and treatment. J Antimicrob Chemother 44:669–674
Walczak DA, Zakrzewski J, Pawelczak D, Grobelski B, Pasieka Z (2013) Evaluation of surgical glove perforation after laparoscopic and open cholecystectomy. Acta Chir Belg 113:423–428
Zaatreh S, Enz A, Klinder A, König T, Mittelmeier L, Kundt G, Mittelmeier W (2016) Prospective data collection and analysis of perforations and tears of latex surgical gloves during primary endoprosthetic surgeries. GMS Hyg Infect Control 11:Doc25
Zbitnew A, Greer K, Heise-Qualtiere J (1988) Conly J (1989) Vinyl versus latex gloves as barriers to transmission of viruses in the health care setting. J Acquir Immune Defic Syndr 2:201–204
Acknowledgements
The authors would like to thank the surgical nurses of the orthopaedic operating theatre for their support during the test series. The authors would like to thank Jessica Hembus for her microscopic support.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All Authors made substantial contributions to conception and design, and/or acquisition of data, and/or analysis and interpretation of data, participated in drafting the article and revising it critically for important intellectual content, approved the submitted version and agreed to be personally accountable for the author’s own contributions and ensured that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, were appropriately investigated, resolved, and the resolution documented in the literature. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors state that there is no conflict of interest.
Ethical approval
Ethics approval for the study was granted by the local ethics committee of the Rostock University Medical Centre (A2020-0236) and data protection requirements were observed.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Enz, A., Klinder, A., Bisping, L. et al. Knot tying in arthroplasty and arthroscopy causes lesions to surgical gloves: a potential risk of infection. Knee Surg Sports Traumatol Arthrosc 31, 1824–1832 (2023). https://doi.org/10.1007/s00167-022-07136-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-022-07136-7