Abstract
Purpose
This study aimed to evaluate the impact on subsequent infections and mortality of an adequate antimicrobial therapy within 48 h after catheter removal in intensive care unit (ICU) patients with positive catheter tip culture.
Methods
We performed a retrospective analysis of prospectively collected data from 29 centers of the OUTCOMEREA network. We developed a propensity score (PS) for adequate antimicrobial treatment, based on expert opinion of 45 attending physicians. We conducted a 1:1 case-cohort study matched on the PS score of being adequately treated. A PS-matched subdistribution hazard model was used for detecting subsequent infections and a PS-matched Cox model was used to evaluate the impact of antibiotic therapy on mortality.
Results
We included 427 patients with a catheter tip culture positive with potentially pathogenic microorganisms. We matched 150 patients with an adequate antimicrobial therapy with 150 controls. In the matched population, 30 (10%) subsequent infections were observed and 62 patients died within 30 days. Using subdistribution hazard models, the daily risk to develop subsequent infection up to Day-30 was similar between treated and non-treated groups (subdistribution hazard ratio [sHR] 1.08, 95% confidence interval [CI] 0.62–1.89, p = 0.78). Using Cox proportional hazard models, the 30-day mortality risk was similar between treated and non-treated groups (HR 0.89, 95% CI 0.45–1.74, p = 0.73).
Conclusions
Antimicrobial therapy was not associated with decreased risk of subsequent infection or death in short-term catheter tip colonization in critically ill patients. Antibiotics may be unnecessary for positive catheter tip cultures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Adequate antimicrobial therapy was not associated with decreased risk of subsequent infection or death in short-term catheter tip colonization in critically ill patients. |
These findings could significantly impact future management strategies when addressing positive catheter tip cultures in critically ill patients. |
Introduction
More than 50% of patients in intensive care units (ICUs) have a central venous catheter (CVC), and the mean device infection-adjusted rate in critically ill patients varies from 0.87 to 4.82 central line-associated bloodstream infections episodes per 1000 CVC-day depending on countries of detection [1,2,3]. Catheter-associated bloodstream infections were associated with a substantially increased attributable mortality [4,5,6] and an additional 15 days of ICU length-of-stay [7]. Colonization of central catheters in the ICU is a frequent phenomenon, affecting more than 8% of intravascular catheter [8], depending on the study. Colonization is not sufficient by itself to define catheter infection but is classically considered as an acceptable surrogate for catheter infection, although poorly correlated with catheter-related infections [9]. Since CVC colonization is the first step of CVC infection, it is likely that CVC colonization predisposes to catheter-related bloodstream infections (CRBSIs) [10]. Therefore, CVC removal is recommended as soon as the CVC is no longer needed to prevent both CVC colonization and infection [11]. To date, there is no uniform approach to the management of colonized catheters, and the consequences in terms of bacteremic or non-bacteremic subsequent infections following catheter removal remain controversial in the literature [12]. In 2009, the Infectious Disease Society of America (IDSA) highlighted the importance of this subject and the need to guide healthcare professionals in initiating an appropriate response to this issue [13].
Up to now, the conclusions of published studies on this topic are limited, because they are (i) heterogeneous, (ii) mostly monocentric and observational, and (iii) mostly describe a single pathogen [10]. A recent national microbiological surveillance study including more than 15,000 positive catheter tips showed that several microorganisms (i.e., Serratia marcescens, Pseudomonas aeruginosa, Staphylococcus aureus and Candida albicans) were associated with an increased risk of subsequent bacteremia [14]. However, due to the retrospective nature of this study without access to clinical data, the role of systemic antibiotic treatment in adults with colonized CVC remains unresolved.
Therefore, we aimed to evaluate the role of systemic antibiotic therapy in patients with a colonized catheter tip with potentially pathogenic microorganisms, assessing the occurrence of bacteremic or non-bacteremic subsequent infections. Preliminary results of this manuscript have been presented at the European Congress of Clinical Microbiology and Infectious Diseases (ECCMID Barcelona, Spain, 27–30 April 2024) [15].
Materials and methods
Study design and data sources
We conducted a matched case-cohort study using the OUTCOMEREA prospective database which has been maintained since 1997 by a total of 32 ICUs in France, comprising 18 in university hospitals. The methodology implemented for data collection and quality control has been described in detail elsewhere [18]. The database protocol was submitted to the Institutional Review Board of the Clermont-Ferrand University Hospital (Clermont-Ferrand, France) which waived the need for informed consent (IRB no. 5891). The OUTCOMEREA database was approved by the French Advisory Committee for Data Processing in Health Research (CCTIRS) and registered by the French National Informatics and Liberty Commission (CNIL, registration no. 8999262), in compliance with French law on electronic data sources. The methods and results of this study are exposed according to the STROBE guidelines [16].
Study population
We included patients from 1st January 1997 to 31st December 2021. Patients were enrolled for the current study if they had a positive quantitative intravascular catheter tip culture, with at least one potentially pathogenic microorganism. Potentially pathogenic microorganisms included S. aureus, Streptococcus spp., Enterococcus spp., Enterobacterales, P. aeruginosa, Acinetobacter spp., and Candida spp. Of note, S. aureus, P. aeruginosa, A. baumannii, other non-fermentative Gram-negative bacteria and Candida spp. were classified as high-risk microorganisms for subsequent infections.
Coagulase-negative staphylococci (except Staphylococcus lugdunensis), Neisseria spp., Corynebacterium spp. (except Corynebacterium JK), Bacillus spp., unspecified Gram-positive cocci were not included. Patients with central venous catheters (CVCs), short-term dialysis catheters and arterial catheters were included. Only the first positive catheter tip culture was considered in this study. Patients with positive blood cultures with a potentially pathogenic microorganism also identified on the catheter tip culture within 48 h before and 48 h after catheter removal (i.e., CRBSI) were excluded. Moreover, patients who died within the first 48 h after catheter removal were also excluded. The follow-up period was 30 days after catheter removal in the ICU.
Definitions, variables of interest and outcomes
Data were extracted from the OUTCOMEREA records of all patients whose intravascular catheters were included in the study and fully reviewed to retrieve demographic, clinical and laboratory data. All study data were obtained from patient files, and no additional tests were performed for the purpose of the current study. The following variables were extracted: severity of illness defined at ICU admission using the Simplified Acute Physiology Score (SAPS) II, age, sex, type of intravascular catheter, duration of catheterization, underlying disease and comorbid conditions, data on mechanical ventilation, duration of hospital stay, symptoms of systemic inflammatory response syndrome (SIRS), immunosuppressive therapy, diagnosis of vessel thrombosis, and characteristics at ICU discharge. For each positive catheter tip culture, we collected data on antimicrobial drugs including type of antibiotic used, duration and day of initiation of antibiotics, organ dysfunction and organ failure defined as Sequential Organ Failure Assessment (SOFA).
Positive intravascular catheter tip culture was defined as a positive quantitative device tip culture showing at least one microorganism yielded ≥ 1000 cfu/ml by vortexing or sonication [17,18,19].
Our variable of interest was an adequate antimicrobial therapy within 48 h after intravascular catheter removal. An adequate antimicrobial therapy was defined as a therapy with at least one antimicrobial with in vitro activity for the microorganism, with adequacy of antimicrobial selection, dosing and administration carefully reviewed for all potentially pathogenic microorganisms by three experts (JRZ, BS and JFT).
Our primary outcome was subsequent infection between 48 h and 30 days after intravascular catheter removal. The 30-day cutoff was chosen based on pathophysiological reasoning, suggesting that it is less likely for a subsequent infection with the same microorganism to be linked to a catheter tip beyond 30 days after its removal. Subsequent infections were defined as infections with the same potentially pathogenic microorganisms detected in the catheter tip culture. Concordance between the microorganism detected in the catheter tip culture and subsequent infections was based on phenotypic microbiological characteristics and the results of antibiotic susceptibility testing (i.e., identical species and antibiogram). The concordance was established by two independent blinded experts (JRZ and BS) who classified these episodes according to infection definitions. In case of disagreement, the opinion of a third expert (JFT) was sought. Infections were classified as subsequent bloodstream infections, surgical site infections (SSI), hospital-acquired pneumonia and urinary tract infection according to European Centre for Disease Prevention and Control (ECDC) definitions. Subsequent infections were assessed at day 30. Our secondary outcomes were 15-day subsequent infections, 15-day mortality and 30-day mortality.
Statistical analysis
Characteristics of patients were described as median (interquartile range) or count (percent) for qualitative and quantitative variables, respectively. Patients receiving an adequate antimicrobial therapy within 48 h were matched with patients without an adequate antimicrobial therapy within 48 h. We performed a propensity score Greedy matching (5 to 1) to select our treated and non-treated patients. We performed a survey with 45 experts to select the most important variables that should be associated with a high antimicrobial treatment probability and included in the propensity score (supplementary methods, eTables 1 and 2). After consultation of 45 experts, the following confounding and prognostic covariates were included in the propensity score: presence of sepsis or septic shock, temperature > 38.5 °C, high SOFA at intravascular catheter removal, time spent in the ICU before intravascular catheter removal, immunosuppression, presence of vascular thrombosis within the first 48 h after intravascular catheter removal, decrease in temperature > 0.5 °C after intravascular catheter removal, and microorganism identified (S. aureus, P. aeruginosa, Candida spp., Streptococcus spp., Enterococcus spp., Acinetobacter spp.). A logistic regression using these variables was performed to develop the propensity score. To assess the quality of matching, we computed standardized mean differences (SMD) for each variable. The risk of developing subsequent infections (at day 30 and day 15) for patients treated within 48 h (versus non-treated patients) was then estimated using Cox proportional subdistribution hazard (Fine and Gray) models stratifying by matched pairs [20]. These models considered mortality as competing event. Proportionality of hazard risk was tested graphically. Further sensitivity analyses for subsequent infections were conducted in patients with catheter tip colonization with high-risk microorganisms or patients with sepsis. To assess 15- and 30-day mortality, we used similar methods, but proportional Cox models were performed without considering competing events. P values < 0.05 were considered to be significant. Statistical analyses were performed using SAS 9.4 (Cary, North Carolina, USA).
Results
Unmatched and matched patients
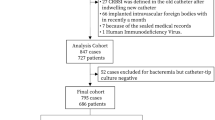
From 15,233 patients with intravascular catheter of the OUTCOMEREA database, we identified 1199 positive intravascular catheter tip cultures; of them, 478 were excluded because they were due to non-potentially pathogenic microorganisms. Moreover, 93 were not first episodes of positive intravascular catheter tip cultures and were, therefore, excluded. Finally, 146 and 55 episodes were excluded because they were associated with concurrent bacteremia and because the patients died within the first 48 h after the positive catheter tip culture, respectively (Fig. 1), leading to a set of 427 patients.
Forty-four percent of these patients (189/427) received an adequate antimicrobial therapy within 48 h after intravascular catheter removal. Overall, 501 microorganisms were identified (eTable 3). The logistic regression model used to develop the propensity score is illustrated in eTable 4 and showed an acceptable calibration and discrimination (AUC [Area Under The Curve] ROC [Receiver Operating Characteristics] 0.675, Hosmer–Lemeshow p value = 0.25). Finally, based on the propensity score, 150 patients with an adequate antimicrobial therapy within 48 h were matched with 150 controls according to the predefined criteria (Table 1, eFigure 1). The matching process was adequate as shown in Table 1 and eFigures 1–2. The characteristics of unmatched and matched cohorts are shown in Table 1.
In the matched population, the median age was 68 (interquartile range [IQR] 53; 77) and 62.1% (n = 182) of patients were male. At the time of positive catheter tip culture, median SOFA score was 5. Patients with sepsis and septic shock were 139 (46.4%) and 39 (13%), respectively. Of note, among the 150 matched patients without antimicrobial within 48 h, 55 (36.7%) received an adequate therapy after a median delay of 4 (IQR 3; 6) days.
Subsequent infections
In the matched population, 30 (10%) subsequent infections were observed, with 15 in the treated and 15 in the non-treated group, after a median delay of 5.5 days (IQR 3; 7). Bacteremia (n = 8) and pneumonia (n = 15) were the most frequently observed subsequent infections. The median time between positive catheter tip culture and subsequent infection was 3 days (IQR 2; 7) in the treated and 6 days (IQR 3; 9) in the non-treated group. The cumulative risk of subsequent infection in matched patients with and without adequate therapy is illustrated in Fig. 2. Microorganisms identified in subsequent infections are illustrated in eTable 5.
Using subdistribution hazard models, the daily risk to develop subsequent infection up to Day-30 was similar between treated and non-treated groups (sHR 1.08, 95% CI [confidence interval] 0.62–1.89, p = 0.78, Fig. 3, eTable 6). Sensitivity analyses including only patients with positive catheter tip with high-risk microorganism (sHR 2.33, 95% CI 0.83–6.54, p = 0.11) or only with sepsis (sHR 0.83, 95% CI 0.46–1.51, p = 0.55) showed similar results. Using proportional subdistribution hazards models, the daily risk to develop subsequent infection up to Day-15 was similar between treated and non-treated groups (sHR 1.18, 95% CI 0.67–2.09, p = 0.57, eTable 6).
Mortality
In the matched population, 62 patients died within 30 days (Table 1), with 33 (22%) in the treated group and 29 (19.3%) in the non-treated group. Using Cox proportional hazard models, the 30-day mortality risk was similar between treated and non-treated groups (HR 0.89, 95% CI 0.45–1.74, p = 0.73, Fig. 3, eTable 6). Sensitivity analyses including only patients with positive catheter tip with high-risk microorganism (HR 1.22, 95% CI 0.51–1.95, p = 0.66) or with sepsis (HR 1.20, 95% CI 0.61–2.38, p = 0.60) showed similar results. We observed similar results using 15-day mortality as an outcome (Fig. 3, eTable 6).
Discussion
In a large multicentre database including more than 400 patients with a positive catheter tip without concurrent bloodstream infection, we found that adequate systemic antibiotic therapy did not decrease the risk of subsequent infection with the same microorganism or death. Results were similar for high-risk microorganisms (e.g., S. aureus) and for patients with sepsis. To the best of our knowledge, it is the largest study exploring this research question.
Several studies suggested that the percentage of catheter with positive quantitative tip culture is correlated with the percentage of CRBSI [9, 18, 21], however, only 17% of patients with positive catheter tip culture has a CRBSI [9]. The decision to perform catheter tip culture is only recommended when infection is suspected [13]. On one hand, infection is frequently suspected in the ICU when the catheter is removed because critically ill patients often present fever, hypothermia or sepsis signs [12, 22, 23]. On the other hand, neither suspicion of infection as the cause of removal nor pathological temperature at removal increased the probability of diagnosing CRBSI.
Concomitant bloodstream infection (BSI) seems to be more frequent and had a higher risk of mortality compared to subsequent BSI [24]. Indeed, Guembe et al., in a large study based on microbiology lab results, showed that concomitant positive blood cultures were observed in 23% of cases [24]. A similar multicentre laboratory-based study from Switzerland showed that the prevalence of CRBSI was 17% [14]. In addition, the prevalence of subsequent BSI was rare ranging from 1.8% (2.8% if coagulase-negative Staphylococci were excluded) [14] and 4.3% [24]. In cohort studies including clinical data and specifically focused on isolated positive catheter tip culture, the risk of subsequent bloodstream infection varied from 1.3 to 53%, with S. aureus, Candida spp. and non-fermentative Gram-negative bacteria carrying the highest risk [12, 14, 24,25,26,27,28,29,30,31,32]. Previous corticosteroid therapy, permanent intravascular prosthesis, underlying immune disease, cancer, the presence of venous thrombosis and acquisition in the ICU setting were associated with a higher likelihood of subsequent BSI [33, 34].
Based on the results of an expert consultation, a large panel of attending physicians and experts selected several of these factors and others (e.g., organ failure and the presence of shock) as determinants for starting antimicrobial therapy in the presence of colonized catheter tip. Our matching strategy was able to balance these factors between treated and untreated patients.
The impact of antimicrobial therapy in decreasing the risk of subsequent infection was evaluated in several studies with conflicting results [12]. On one hand, several studies showed that antimicrobial therapy for positive catheter tip culture could have a positive impact on subsequent infections. For S. aureus-positive tip cultures, cohort studies [25, 26, 35] showed that antibiotic therapy decrease the risk of subsequent BSI. In the Ekkelenkamp et al. study the risk of subsequent BSI for S. aureus-positive catheter tip culture was 24% and early antimicrobial therapy significantly reduced the risk [27]. However, the authors did not assess if blood cultures were systematically drawn within 48 h and may have missed several cases of CRBSI. Similar results were observed for A. baumannii and for P. aeruginosa-positive catheter tip culture in single-center studies [30, 32].
On the other hand, several studies showed less impact of antimicrobial therapy on subsequent infections in patients with positive catheter tip with S. aureus, Candida spp. and Gram-negative microorganisms [28, 31, 36, 37]. To our knowledge, no large multicentre study investigated the impact of antimicrobial treatment of positive catheter tip cultures on mortality. Our results suggested that antimicrobial treatment may be unnecessary for patients with positive catheter tips with high-risk microorganisms but without concomitant positive blood cultures. This finding could contribute to antimicrobial stewardship efforts, potentially reducing antibiotic overuse in critically ill patients.
Our study has several limitations. First, information bias may have influenced the results. In the available articles, the reason for culturing catheter tip was not detailed. We made a specific effort, considering all patient characteristics upon ICU admission and their clinical status at the time of catheter tip removal. However, it is important to note that local signs or symptoms of infection, such as purulence or pain, were neither recorded nor considered in the matching process. Moreover, reasons for catheter removal were not routinely collected. While local signs have been linked to an increased likelihood of CRBSI, they have never been reported as a risk factor for subsequent infections [23]. Other unmeasured confounders may also have been overlooked. Second, patients were not monitored for new infections after discharge from the ICU. In studies conducted outside the ICU, the follow-up duration was longer, potentially explaining for the higher risk of subsequent infections. Third, S. aureus, the only microorganism for which a reduced risk of subsequent infection with antimicrobial treatment was observed in several articles, represented only 10% of the cases in our cohort. Therefore, it is conceivable that our study was underpowered to detect an increased risk of poor outcome without therapy for a specific microorganism. In this context, we observed a higher risk of subsequent infections, although it did not reach statistical significance, when considering only high-risk microorganisms. However, our study population included only microorganisms which showed a priori an increased risk for subsequent infections and excluded low-risk microorganisms (e.g., coagulase-negative Staphylococci). Fourth, patients who died in the first 48 h were excluded from the analysis because they were not exposed to antibiotics. We could not exclude that this cohort represented an extreme of the unfavorable association between the exposure and our secondary outcome. Finally, we evaluated only the impact of early treatment of positive catheter tips without assessing the impact of a delayed antimicrobial therapy that may influence the occurrence of subsequent infections. An additional post hoc analysis including patients who received an adequate therapy between 48 and 96 h or without an adequate treatment within the first 96 h showed similar results (supplementary material).
Conclusions
Using a large multicentre cohort, we showed that early antimicrobial therapy was not associated with decreased risk of subsequent infection or death in short-term catheter tip colonization in critically ill patients. Antibiotics may be probably avoided for positive catheter tip cultures.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the OUTCOMEREA organization on reasonable request.
References
Patel PR, Weiner-Lastinger LM, Dudeck MA, Fike LV, Kuhar DT, Edwards JR et al (2022) Impact of COVID-19 pandemic on central-line-associated bloodstream infections during the early months of 2020, National Healthcare Safety Network. Infect Control Hosp Epidemiol 43(6):790–793
Teja B, Bosch NA, Diep C, Pereira TV, Mauricio P, Sklar MC et al (2024) Complication rates of central venous catheters: a systematic review and meta-analysis. JAMA Intern Med 184(5):474–482
Rosenthal VD, Yin R, Myatra SN, Memish ZA, Rodrigues C, Kharbanda M et al (2023) Multinational prospective study of incidence and risk factors for central-line-associated bloodstream infections in 728 intensive care units of 41 Asian, African, Eastern European, Latin American, and Middle Eastern countries over 24 years. Infect Control Hosp Epidemiol 28:1–11. https://doi.org/10.1017/ice.2023.69
Ziegler MJ, Pellegrini DC, Safdar N (2015) Attributable mortality of central line associated bloodstream infection: systematic review and meta-analysis. Infection 43(1):29–36
Adrie C, Garrouste-Orgeas M, Ibn Essaied W, Schwebel C, Darmon M, Mourvillier B et al (2017) Attributable mortality of ICU-acquired bloodstream infections: impact of the source, causative micro-organism, resistance profile and antimicrobial therapy. J Infect 74(2):131–141
Tabah A, Buetti N, Staiquly Q, Ruckly S, Akova M, Aslan AT et al (2023) Epidemiology and outcomes of hospital-acquired bloodstream infections in intensive care unit patients: the EUROBACT-2 international cohort study. Intensive Care Med 49(2):178–190
Yu KC, Jung M, Ai C (2023) Characteristics, costs, and outcomes associated with central-line-associated bloodstream infection and hospital-onset bacteremia and fungemia in US hospitals. Infect Control Hosp Epidemiol 44(12):1920–1926. https://doi.org/10.1017/ice.2023.132
Buetti N, Souweine B, Mermel L, Mimoz O, Ruckly S, Loiodice A et al (2021) Concurrent systemic antibiotics at catheter insertion and intravascular catheter-related infection in the ICU: a post hoc analysis using individual data from five large RCTs. Clin Microbiol Infect 27(9):1279–1284
Rijnders BJ, Van Wijngaerden E, Peetermans WE (2002) Catheter-tip colonization as a surrogate end point in clinical studies on catheter-related bloodstream infection: how strong is the evidence? Clin Infect Dis 35(9):1053–1058
Buetti N, Timsit JF (2019) Management and prevention of central venous catheter-related infections in the ICU. Semin Respir Crit Care Med 40(4):508–523
Timsit JF, Baleine J, Bernard L, Calvino-Gunther S, Darmon M, Dellamonica J et al (2020) Expert consensus-based clinical practice guidelines management of intravascular catheters in the intensive care unit. Ann Intensive Care 10(1):118
Timsit JF, Rupp M, Bouza E, Chopra V, Karpanen T, Laupland K et al (2018) A state of the art review on optimal practices to prevent, recognize, and manage complications associated with intravascular devices in the critically ill. Intensive Care Med 44(6):742–759
Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP et al (2009) Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 49(1):1–45
Buetti N, Lo Priore E, Atkinson A, Kronenberg A, Marschall J, Swiss Centre for Antibiotic R (2018) Low incidence of subsequent bacteraemia or fungaemia after removal of a colonized intravascular catheter tip. Clin Microbiol Infect 24(5):548 e1-548 e3
Buetti N, Zahar JR, Adda M, Ruckly S, Bruel C, Schwebel7 C, Darmon M, Adrie C, Cohen Y, Siami S, Laurent V, Souweine B, Timsit JF. Management of positive catheter tip cultures without concurrent bloodstream infections in critically ill patients, prognosis and risk of subsequent infection: a case-cohort study from the OUTCOME REA network. Abstract number 01249. 34th European congress of clinical microbiology and infectious diseases
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP et al (2007) Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 335(7624):806–808
Sherertz RJ, Raad II, Belani A, Koo LC, Rand KH, Pickett DL et al (1990) Three-year experience with sonicated vascular catheter cultures in a clinical microbiology laboratory. J Clin Microbiol 28(1):76–82
Safdar N, Fine JP, Maki DG (2005) Meta-analysis: methods for diagnosing intravascular device-related bloodstream infection. Ann Intern Med 142(6):451–466
Brun-Buisson C, Abrouk F, Legrand P, Huet Y, Larabi S, Rapin M (1987) Diagnosis of central venous catheter-related sepsis. Critical level of quantitative tip cultures. Arch Intern Med 147(5):873–877
Fine JPGR (1999) A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999(94):496–509
de Grooth HJ, Timsit JF, Mermel L, Mimoz O, Buetti N, du Cheyron D et al (2020) Validity of surrogate endpoints assessing central venous catheter-related infection: evidence from individual- and study-level analyses. Clin Microbiol Infect 26(5):563–571
Timsit JF, Lugosi M, Minet C, Schwebel C (2011) Should we still need to systematically perform catheter culture in the intensive care unit? Crit Care Med 39(6):1556–1558
Buetti N, Ruckly S, Lucet JC, Bouadma L, Garrouste-Orgeas M, Schwebel C et al (2020) Local signs at insertion site and catheter-related bloodstream infections: an observational post hoc analysis using individual data of four RCTs. Crit Care 24(1):694
Guembe M, Rodriguez-Creixems M, Martin-Rabadan P, Alcala L, Munoz P, Bouza E (2014) The risk of catheter-related bloodstream infection after withdrawal of colonized catheters is low. Eur J Clin Microbiol Infect Dis 33(5):729–734
Zafar U, Riederer K, Khatib R, Szpunar S, Sharma M (2009) Relevance of isolating Staphylococcus aureus from intravascular catheters without positive blood culture. J Hosp Infect 71(2):193–195
Ruhe JJ, Menon A (2006) Clinical significance of isolated Staphylococcus aureus central venous catheter tip cultures. Clin Microbiol Infect 12(9):933–936
Ekkelenkamp MB, van der Bruggen T, van de Vijver DA, Wolfs TF, Bonten MJ (2008) Bacteremic complications of intravascular catheters colonized with Staphylococcus aureus. Clin Infect Dis 46(1):114–118
Perez-Parra A, Munoz P, Guinea J, Martin-Rabadan P, Guembe M, Bouza E (2009) Is Candida colonization of central vascular catheters in non-candidemic, non-neutropenic patients an indication for antifungals? Intensive Care Med 35(4):707–712
Park KH, Kim SH, Song EH, Jang EY, Lee EJ, Chong YP et al (2010) Development of bacteraemia or fungaemia after removal of colonized central venous catheters in patients with negative concomitant blood cultures. Clin Microbiol Infect 16(6):742–746
Apisarnthanarak A, Apisarnthanarak P, Warren DK, Fraser VJ (2012) Is central venous catheter tip colonization with Pseudomonas aeruginosa a predictor for subsequent bacteremia? Clin Infect Dis 54(4):581–583
van Eck van der Sluijs A, Oosterheert JJ, Ekkelenkamp MB, Hoepelman IM, Peters EJ (2012) Bacteremic complications of intravascular catheter tip colonization with Gram-negative micro-organisms in patients without preceding bacteremia. Eur J Clin Microbiol Infect Dis 31(6):1027–1033
Apisarnthanarak A, Apisarnthanarak P, Warren DK, Fraser VJ (2011) Is central venous catheter tips’ colonization with multi-drug resistant Acinetobacter baumannii a predictor for bacteremia? Clin Infect Dis 52(8):1080–1082
Timsit JF, Farkas JC, Boyer JM, Martin JB, Misset B, Renaud B et al (1998) Central vein catheter-related thrombosis in intensive care patients: incidence, risks factors, and relationship with catheter-related sepsis. Chest 114(1):207–213
Buetti N, Lo Priore E, Sommerstein R, Atkinson A, Kronenberg A, Marschall J et al (2018) Epidemiology of subsequent bloodstream infections in the ICU. Crit Care 22(1):259
Hetem DJ, de Ruiter SC, Buiting AGM, Kluytmans J, Thijsen SF, Vlaminckx BJM et al (2011) Preventing Staphylococcus aureus bacteremia and sepsis in patients with Staphylococcus aureus colonization of intravascular catheters: a retrospective multicenter study and meta-analysis. Medicine (Baltimore) 90(4):284–288
Lopez-Medrano F, Lora-Tamayo J, Fernandez-Ruiz M, Losada I, Hernandez P, Cepeda M et al (2016) Significance of the isolation of Staphylococcus aureus from a central venous catheter tip in the absence of concomitant bacteremia: a clinical approach. Eur J Clin Microbial Infect Dis 35(11):1865–1869
Mrozek N, Lautrette A, Aumeran C, Laurichesse H, Forestier C, Traore O et al (2011) Bloodstream infection after positive catheter cultures: what are the risks in the intensive care unit when catheters are routinely cultured on removal? Crit Care Med 39(6):1301–1305
Acknowledgements
We thank the OUTCOMEREA network. The authors thank Céline Féger, MD (EMIBiotech) for her editorial support.
Scientific Committee: Jean-François Timsit (Medical and Infectious Diseases ICU, Bichat-Claude Bernard Hospital, Paris, France; UMR 1137 Inserm –Paris Diderot university IAME, F75018, Paris); Elie Azoulay (Medical ICU, Saint Louis Hospital, Paris, France); Maïté Garrouste-Orgeas (Paliative care, Institut franco Britanique, Paris, France); Jean-Ralph Zahar (Infection Control Unit, Angers Hospital, Angers, France); Bruno Mourvillier (Medical ICU, CHU Reims,France); Michael Darmon (Medical ICU, APHP Saint Louis hospital Paris, France); Niccolò Buetti (Geneva, Switzerland). Biostatistical and Information System Expertise: Jean-Francois Timsit (Medical and Infectious Diseases ICU, Bichat-Claude Bernard Hospital, Paris, France; UMR 1137 Inserm –Paris Diderot university IAME, F75018, Paris); Corinne Alberti (Medical Computer Sciences and Biostatistics Department, Robert Debré Hospital, Paris, France); Stephane Ruckly (OUTCOMEREA organization and Inserm UMR 1137 IAME, F75018, Paris); Sébastien Bailly (Grenoble university hospital Inserm UMR 1137 IAME, F75018, Paris) and Aurélien Vannieuwenhuyze (Tourcoing, France). Investigators of the OUTCOMEREA Database: Christophe Adrie (ICU, CH Melun, and Physiology, Cochin Hospital, Paris, France); Carole Agasse (medical ICU, university hospital Nantes, France); Bernard Allaouchiche (ICU, Hospices civils de lyon, Lyon sud, Lyon, France); Olivier Andremont (ICU, Bichat Hospital, Paris, France); Pascal Andreu (CHU Dijon, Dijon, France); Laurent Argaud (Medical ICU, Hospices Civils de Lyon, Lyon, France); Elie Azoulay (Medical ICU, Saint Louis Hospital, Paris, France); Francois Barbier (medical-surgical ICU, Orleans, France), Jean-Pierre Bedos (ICU, Versailles Hospital, Versailles, France); Jérome Bedel (ICU, Versailles Hospital, Versailles, France), Asael Berger (ICU, CH Haguenau, France) ; Julien Bohé (ICU, Hôpital Pierre Benite, Lyon France), Lila Bouadma (ICU, Bichat Hospital, Paris, France); Jeremy Bourenne (Réanimation des urgences, Timone-2; APHM, Marseille, France); Noel Brule (medical ICU, university hospital Nantes, France); Frank Chemouni (Grand Hôpital de l’Est Francilien Site Marne La vallée ; Polyvalent ICU, 77600 Jossigny Polyvalent ICU) ; Julien Carvelli (Réanimation des urgences, Timone-2; APHM, Marseille, France); Martin Cour Medial ICU, Edouard Heriot hospital, Lyon France), Michael Darmon (ICU, APHP St louis, Paris France); Julien Dessajan (ICU, Bichat Hospital, Paris, France), Claire Dupuis (ICU, G Montpied Hospital, Clermont-Ferrand, France), Etienne de Montmollin (ICU, Bichat Hospital, Paris, France), Marc Doman (ICU AP HP Bichat, paris France) ; Loa Dopeux (ICU, G Montpied Hospital, Clermont-Ferrand, France); Anne-Sylvie Dumenil (Antoine Béclère Hospital, Clamart, France); Claire Dupuis (Bichat hospital and UMR 1137 Inserm –Paris Diderot university IAME, F75018, Paris, France), Jean-Marc Forel (AP HM, Medical ICU, Hôpital Nord Marseille), Marc Gainnier (Réanimation des urgences, Timone-2; APHM, Marseille, France), Charlotte Garret (medical ICU, university hospital Nantes, France); Louis-Marie Galerneau (CHU A Michallon, Grenoble, France), Dany Goldgran-Tonedano ( CH le Raincy-Montfermeil; France); Steven Grangé (ICU, CHU Rouen, France), Antoine Gros (ICU, Versailles Hospital, Versailles, France), Hédia Hammed (CH le Raincy-Montfermeil) ; Akim Haouache (Surgical ICU, H Mondor Hospital, Creteil, France); Tarik Hissem (ICU, Eaubonne, France), Vivien Hong Tuan Ha (ICU, CH Meaux, France); Sébastien Jochmans (ICU, CH Melun); Jean-Baptiste Joffredo (ICU, G Montpied Hospital, Clermont-Ferrand, France); Hatem Kallel (ICU, Cayenne General Hospital, Cayenne, France); Guillaume Lacave (ICU, Versailles Hospital, Versailles, France), Virgine Laurent (ICU, Versailles Hospital, Versailles, France), Alexandre Lautrette (ICU, G Montpied Hospital, Clermont-Ferrand, France); Clément Le bihan (ICU, Bichat Hospital, Paris, France), Virgine Lemiale (Medical ICU, Saint Louis Hospital, Paris, France); David Luis (Médecine intensive et réanimation, CH Simone Veil, Beauvais, France), Guillaume Marcotte (Surgical ICU, Hospices Civils de Lyon, Lyon, France); Jordane Lebut (ICU, Bichat Hospital, Paris, France); Bruno Mourvillier (ICU, Bichat Hospital, Paris, France); Benoît Misset (ICU, Saint-Joseph Hospital, Paris, France); Bruno Mourvillier (ICU, Medical ICU, Reims France); Mathild Neuville (ICU, Foch Hospital, Paris, France) ; Laurent Nicolet (medical ICU, university hospital Nantes, France); Johanna Oziel (Medico-surgical ICU, hôpital Avicenne APHP, Bobigny, France), Laurent Papazian (Hopital Nord, Marseille, France), Juliette Patrier (ICU, Bichat Hospital, Paris, France), Benjamin Planquette (pulmonology ICU, George Pompidou hospital Hospital, Paris, France); Aguila Radjou (ICU, Bichat Hospital, Paris, France), Marie Simon (Medial ICU, Edouard Heriot hospital, Lyon France), Romain Sonneville (ICU, Bichat Hospital, Paris, France), Jean Reignier (medical ICU, university hospital Nantes, France); Bertrand Souweine (ICU, G Montpied Hospital, Clermont-Ferrand, France); Carole Schwebel (ICU, A Michallon Hospital, Grenoble, France); Shidasp Siami (ICU, Eaubonne, France); Romain Sonneville (ICU, Bichat Hospital, Paris, France); Michael thy (ICU, Bichat Hospital, Paris, France) ; (Gilles Troché (ICU, Antoine Béclère Hospital, Clamart, France); Fabrice Thiollieres (ICU, Hospices civils de lyon, Lyon sud, Lyon, France) ; Guillaume Thierry (ICU, St Etienne, France); Michael Thy (ICU, APHP, Bichat France) ; Guillaume Van Der Meersch (Medical Surgical ICU, university hospital Avicenne), Marion Venot (Medical ICU, Saint Louis Hospital, Paris, France); Florent Wallet (ICU, Hospices civils de lyon, Lyon sud, Lyon, France); Sondes Yaacoubi (CH le Raincy-Montfermeil); Olivier Zambon (medical ICU, university hospital Nantes, France); Jonathan Zarka (reanimation polyvalente, centre hospitalier de marne la Vallee, France). Kévin grapin (ICU, G Montpied Hospital, Clermont-Ferrand, France), Francois thouy (ICU, G Montpied Hospital, Clermont-Ferrand, France), Laure Calvet (ICU, G Montpied Hospital, Clermont-Ferrand, France), kevin Grapin (ICU, G Montpied Hospital, Clermont-Ferrand, France), guillaume laurichesse (ICU, G Montpied Hospital, Clermont-Ferrand, France), Martin COUR (Medical ICU, Hospices Civils de Lyon, Lyon, France), Neven STEVIC (Medical ICU, Hospices Civils de Lyon, Lyon, France). Study Monitors: Mireille Adda, Vanessa Vindrieux, Marion Provent, Pauline Enguerrand, Vincent Gobert, Stéphane Guessens, Helene Merle, Nadira Kaddour, Boris Berthe, Samir Bekkhouche, Kaouttar Mellouk, Mélaine Lebrazic, Carole Ouisse, Diane Maugars, Christelle Aparicio, Igor Theodose, Manal Nouacer, Veronique Deiler, Fariza Nait Sidenas, Myriam Moussa, Atika Mouaci, Nassima Viguier.
Funding
Open access funding provided by University of Geneva. NB received a Mobility grant from the Swiss National Science Foundation (Grant number: P400PM_183865).
Author information
Authors and Affiliations
Consortia
Contributions
NB, JRZ, SR, BS and JFT designed and conceptualized the study. All the other coauthors acquired the data in their ICUs. SR, NB and JFT did the statistical analysis. SR performed the data curation. NB, SR and JFT analyzed and interpreted the data. NB, JRZ and JFT drafted the manuscript. All the authors critically reviewed the manuscript and approved the final report.
Corresponding author
Ethics declarations
Conflicts of interest
J-FT reported advisory boards participation for Merck, Gilead, Beckton-Dickinson, Pfizer, Shionogi, Roche diagnostic, Advanz Pharma, research grants from Merck, Pfizer, Thermofischer. MD received support from the “Société de reanimation de langue francaise” and “Groupe de Recherche en Réanimation respiratoire et Onco-hematologique”.
Ethics approval
The database protocol was submitted to the Institutional Review Board of the Clermont-Ferrand University Hospital (Clermont-Ferrand, France) which waived the need for informed consent (IRB no. 5891). The OUTCOMEREA database was approved by the French Advisory Committee for Data Processing in Health Research (CCTIRS) and registered by the French National Informatics and Liberty Commission (CNIL, registration no. 8999262), in compliance with French law on electronic data sources.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The members of the OUTCOMEREA study group are listed in the Acknowledgement section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Buetti, N., Zahar, JR., Adda, M. et al. Treatment of positive catheter tip culture without bloodstream infections in critically ill patients. A case-cohort study from the OUTCOMEREA network. Intensive Care Med (2024). https://doi.org/10.1007/s00134-024-07498-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00134-024-07498-1