Abstract
Purpose
We assessed long-term outcomes in acutely admitted adult patients with delirium treated in intensive care unit (ICU) with haloperidol versus placebo.
Methods
We conducted pre-planned analyses of 1-year outcomes in the Agents Intervening against Delirium in the ICU (AID-ICU) trial, including mortality and health-related quality of life (HRQoL) assessed by Euroqol (EQ) 5-dimension 5-level questionnaire (EQ-5D-5L) index values and EQ visual analogue scale (EQ VAS) (deceased patients were assigned the numeric value zero). Outcomes were analysed using logistic and linear regressions with bootstrapping and G-computation, all with adjustment for the stratification variables (site and delirium motor subtype) and multiple imputations for missing HRQoL values.
Results
At 1-year follow-up, we obtained vital status for 96.2% and HRQoL data for 83.3% of the 1000 randomised patients. One-year mortality was 224/501 (44.7%) in the haloperidol group versus 251/486 (51.6%) in the placebo group, with an adjusted absolute risk difference of − 6.4%-points (95% confidence interval [CI] − 12.8%-points to − 0.2%-points; P = 0.045). These results were largely consistent across the secondary analyses. For HRQoL, the adjusted mean differences were 0.04 (95% CI − 0.03 to 0.11; P = 0.091) for EQ-5D-5L-5L index values, and 3.3 (95% CI − 9.3 to 17.5; P = 0.142) for EQ VAS.
Conclusions
In acutely admitted adult ICU patients with delirium, haloperidol treatment reduced mortality at 1-year follow-up, but did not statistically significantly improve HRQoL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In this pre-planned 1-year follow-up of the AID-ICU trial, we found that treatment with haloperidol in critically ill patients with delirium reduced long-term mortality but did not seem to improve the long-term outcome of health-related quality of life. |
Introduction
Delirium is an acute change in attention and awareness which develops over a short time and is associated with additional cognitive deficits such as memory loss, disorientation, or perceptual disturbances [1]. Delirium is the most frequent form of acute brain dysfunction in intensive care unit (ICU) patients [2, 3]. Approximately half of adult patients in the ICU experience delirium during their critical illness, with a higher occurrence in patients receiving mechanical ventilation [2]. Delirium has significant adverse implications, ranging from agitation, pulling out lines and tubes, prolonged time on mechanical ventilation to increased mortality [4, 5]. Moreover, delirium in the ICU is a risk factor for long-term impairments, including cognitive impairment and functional decline. These long-term sequelae may impact health-related quality of life (HRQoL) and mortality [5,6,7,8]. It is currently unclear if the possible benefits from antipsychotic treatment of ICU delirium, e.g. decreased agitation or delirium severity, also impacts long-term HRQoL related outcomes as less disability or cognitive dysfunction, or mortality [9,10,11].
Prevention and treatment of delirium in the ICU continue to be a clinical challenge [9, 10, 12, 13]. Currently, haloperidol is the most frequently used pharmacological agent to treat delirium in the ICU, but the evidence for its effect has been sparse and inconclusive [14, 15]. The recent Agents Intervening against Delirium in the ICU (AID-ICU trial) assessed the benefits and harms of haloperidol versus placebo in acutely admitted adult ICU patients with delirium [16]. The trial found no statistically significant difference in the primary outcome days alive and out of hospital within 90 days after randomisation, but lower mortality at 90 days was observed among patients in the haloperidol group. The pre-planned, Bayesian analysis found high probabilities of benefit and low probabilities of harm with haloperidol treatment of delirium in adult patients admitted to the ICU, specific for the primary outcome with probabilities of 92% for days alive and out of hospital to day 90 [16, 17].
In this pre-planned 1-year follow-up study of the AID-ICU trial, we report the long-term effects of haloperidol versus placebo on mortality and HRQoL. We hypothesised that haloperidol would reduce mortality and increase quality of life [18].
Methods
Study design
The AID-ICU trial was a multicentre, randomised, blinded, parallel-group, placebo-controlled trial where eligible patients were randomly assigned in a 1:1 ratio to receive either haloperidol or placebo (isotonic saline). The AID-ICU trial complied with the Declaration of Helsinki and was approved by the relevant health authorities, ethics committees and data-protection agencies in participating countries [19, 20]. The AID-ICU trial protocol, statistical analysis plan, and short-term outcomes have been published elsewhere [16, 19, 21]. This study is a pre-planned 1-year follow-up of the AID-ICU trial with a separate protocol and statistical analysis plan published before randomisation completion [18], with some deviations from the published protocol (outlined with rationales in the electronic supplementary material [ESM] 1). This manuscript is reported according to the Consolidated Standards of Reporting Trials (CONSORT) 2010 Statement (ESM 2) [22].
Trial population and intervention
From June 2018 to April 2022, 1000 patients were enrolled in the AID-ICU trial at 16 ICUs in Denmark, Finland, the United Kingdom, and Italy. Eligibility criteria were adult critically ill patients who were acutely admitted to the ICU and been diagnosed with delirium, with either Confusion Assessment Method for the ICU (CAM-ICU) or the Intensive Care Delirium Screening Checklist (ICDSC) [23, 24]. The patients were randomised to receive haloperidol or placebo (isotonic saline) corresponding to 2.5 mg haloperidol three times daily and additional as-needed doses to a maximum daily dose of 20 mg. Patients received study drug if they were delirious in the ICU for a maximum of 90 days. For further details, see ESM 1 [16, 19].
Outcomes
The pre-specified outcomes of this follow-up study were all-cause mortality and HRQoL 1 year after randomisation. Additional outcomes were differences in HRQoL between survivors only.
To assess HRQoL, we used the EuroQol 5-dimension 5-level questionnaire (EQ-5D-5L) and the EuroQol Visual Analogue Scale (EQ VAS) [25]. EQ-5D-5L is a descriptive system evaluating five dimensions of health. The patients assess their mobility, self-care, usual activity, pain/discomfort, and anxiety/depression and choose the most applicable of five levels ranging from no problems, slight problems, moderate problems, severe problems, and unable to/extreme problems. The result of the questionnaire represents an individual health state profile, also called EQ-5D-5L profile which can be converted into a single summary score; an EQ-5D-5L index value. The index value reflects how well people perceive themselves according to the preference of the general population of a country/region [25]. For this study, we used the Danish and English value set to calculate EQ-5D-5L index value set [26,27,28,29]. Since a nation-specific value set for Finland does not exist, the Danish value set was used for these patients. There were no survivors at 1 year follow-up in Italy.
The EQ-5D-5L index values are anchored at 1.0, corresponding to ‘perfect health’ to a value of minus 1.0. A value of 0 corresponds to a self-reported health status ‘as bad as being dead’, and a value < 0 corresponds to a self-reported health status ‘worse than death’ [30]. The lowest index value depends on the value sets used; for Denmark, index values range from − 0.757 to 1 [31]. EQ-5D-5L is a validated, recommended instrument for assessing HRQoL in ICU settings [32]. EQ VAS is an overall measure of self-reported health, with a score ranging from 0 (worst possible health) to 100 (best imaginable health) on that specific day.
Patients who died within the 1-year follow-up were assigned 0 for the HRQoL values, corresponding to a health state as bad as being dead for EQ-5D-5L index values and to the lowest possible value for EQ-VAS [25, 30].
Data collection
A standard operating procedure was followed to increase follow-up rate and secure uniform data collection (ESM 1). Primary investigators at each site collected vital status and the date of death of non-survivors from medical records. Survivors were contacted to obtain HRQoL by phone by trained research personnel, who were blinded to the allocation. Several attempts were made to contact patients to minimise loss of follow-up. National investigators were responsible for collecting follow-up data in their countries. Patients were interviewed in their native language. In cases where patients were unable to participate in the interview, relatives performed the HRQoL on the patient's behalf using the tool’s proxy version.
Statistical analysis
The primary analyses were conducted in the intention-to-treat (ITT) population, defined as all randomised patients who received the intervention and who had consented to use the data. We present descriptive baseline data stratified by treatment allocation (haloperidol/placebo) and survival/response status. Numerical data were summarised with median and interquartile range (IQR) and categorical data were summarised with numbers and percentages.
Analyses
The primary analyses were adjusted for stratification variables: site and delirium motor subtypes (hyperactive or hypoactive) at randomisation. We conducted secondary analyses without adjustment and with adjustment for the following additional variables: stratification, sex, age (< 69 years versus ≥ 69 years) and Simplified Mortality Score for the Intensive Care Unit (SMS-ICU; < 25 versus ≥ 25) [18, 33]. Finally, we analysed outcomes in the per-protocol population, excluding patients with one or more major protocol violations (ESM 1).
We used logistic and linear regression models with G-computation and bootstrapping (50,000 bootstrap resamples) to calculate sample average treatment effects presented on the absolute (risk differences [RDs] and mean differences [MDs]) and relative (ratios of means [RoMs] and risk ratios [RRs]) scales with 95% confidence intervals (CIs) [34]. P-values were derived from the G-computation and bootstrapping procedure for binary outcomes and the Kryger-Jensen and Lange test [35] for continuous outcomes. For analyses conducted in survivors only, P-values were derived from the G-computation and bootstrapping procedure as data were not zero-inflated in this population. Mortality analyses were supplemented with a Kaplan–Meier plot and calculation of a hazard ratio from a Cox proportional hazards model adjusted for stratification variables.
To assess the impact of the intervention in individual EQ-5D-5L domains we used proportional odds logistic regression models to calculate the overall effect in a domain, the results were reported as ordinal odds ratios (OR) with 95% CIs. To estimate differences between each cut-off in a domain and mortality we used logistic regression models to calculate OR with 95% CIs. These analyses were performed in the multiply imputed dataset for all patients, survivors only and the per-protocol population.
Missing data handling
For mortality we used complete case analysis due to limited missing data (2.5%). For the HRQoL outcomes, 14% had missing data, exceeding the predefined threshold of 5%, and Little's test indicated that data were not missing completely at random (P < 0.001). Consequently, we used multiple imputations with the predictive mean matching method to generate 50 imputed datasets separately in each treatment group. The imputation model included the stratification variables (site and delirium motor subtype) and baseline values (age, sex, admission type, hematologic disease or cancer, risk factors for delirium, and individual components of SMS-ICU) and reported outcomes (days alive and out of hospital, hospital length of stay, days alive without delirium or coma, days alive without mechanical ventilation, serious adverse reactions to haloperidol, use of escape medication and days with escape medication), except 90-day mortality which was not included due to high correlation with 1-year mortality. To assess the impact of missing data, we conducted best–worst and worst-best-case scenario sensitivity analyses using the mean ± 2 standard deviations (SD) of the EQ-5D-5L values calculated in all surviving patients with complete data with imputed values truncated to the possible range of values for each outcome.
We report 95% CIs and consider P-values below 5% as statistically significant. All analyses were performed using R 4.2.3 (R Core Team, Foundation for Statical Computing, Vienna, Austria).
Results
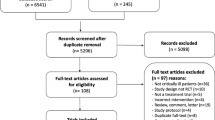
One thousand patients were randomised to the AID-ICU trial. Thirteen patients never received any trial medication and were therefore excluded from the analysis, leaving a total of 987 included in this follow-up study. A total of 25 patients withdrew consent before 1-year follow-up. For HRQoL, there were 138 non-respondents: 72 (14.4%) in the haloperidol group and 66 (13.6%) in the placebo group (Fig. 1). The baseline characteristics between the two intervention groups were well-balanced within the survival and respondence status strata (Table 1). Comparing groups based on survival and respondence status, some differences were present. Non-survivors were older and appeared to have a more coexisting conditions (hematologic cancer) and higher predicted mortality risk. Non-respondents appeared to have more baseline risk factors for delirium (e.g., substance abuse, smoking and stroke within six months) (Table 1).
Consort diagram. Patient flow in the AID-ICU trial. Details up to 90 days were presented in the primary report [16]. 1000 patients were randomised in the AID-ICU trial. Thirteen patients never received any trial medication and were excluded from the analysis. Twenty-five patients withdrew consent before 1-year follow-up. The primary HRQoL analyses were done in the ITT population (n = 987) with deceased patients assigned zero and missing data (n = 165 for EQ-5D-5L index values and n = 163 for EQ VAS scores) multiply imputed
1-year mortality
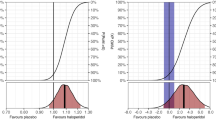
We obtained 1-year vital status from 962 of the 987 (97.5%) patients. At 1-year 214 of 491 patients (43.6%) in the haloperidol group had died compared with 236 of 471 (50.1%) patients in the placebo group (adjusted absolute difference − 6.4%-points (95% CI − 12.8%-points to − 0.2%-points; P = 0.045) (Table 2). The secondary analyses were similar to the primary analysis (ESM 1, Table S1). The Kaplan–Meier survival curves are presented in Fig. 2a with the adjusted Cox-regression hazard ratio estimate.
1-year survival curve and heatmap. a Survival curves in the two groups at one year (day 365). Patients who withdrew consent for further data or were lost to follow-up were censored at the time of withdrawal or loss to follow-up. Cox regression adjusted for stratification variables found a hazard ratio (HR) 0.81 (95% CI 0.67–0.97). b The distribution of HRQoL (EQ-5D-5L and EQ-VAS) data are shown as a heatmap in all patients after multiple imputations; non-survivors were assigned zero. The colour scheme: red represents worse outcomes, and blue represents better outcomes. The horizontal axes represent the cumulated proportion of the patients scoring at or below the value on the secondary axes and represent the two tools used for HRQoL; EQ-5D-5L index value from below 0 (corresponding to health states valued worse than death) to 1 and EQ VAS from 0 to 100. Similar heatmaps for survivors only are presented in the ESM 1
Health-related quality of life
We obtained 1-year HRQoL data from 824 of 987 (83.5%). The proportions of relatives answering the HRQoL questionnaire on behalf of patients was 5 of 205 (2.4%) in the haloperidol group and 6 of 169 (3.6%) in the placebo group.
At 1-year follow-up the median EQ-5D-5L index value were 0.3 (IQR 0–0.9) in the haloperidol group and 0 (IQR 0–0.8) in the placebo group, resulting in an adjusted MD of 0.04 (95% CI − 0.03 to 0.11; P = 0.091, Table 2). Median EQ VAS score were 25.0 in the haloperidol group versus 0 in the placebo group, resulting in an adjusted MD of 3.3 (95% CI − 9.3 to 17.5; P = 0.142) (Table 2 and Fig. 2b). The results of the sensitivity analyses were consistent with the primary analysis. Best–worst and worst–best sensitivity analyses showed that missing data from non-responders could have influenced the results (ESM 1, Table S2).
We found similar results in survivors only, see Table 2, and also within each single subdomain of EQ-5D-5L (Fig. 3 and ESM 1, Table S2). Descriptive data of EQ-5D-5L in survivors only are presented in ESM 1.
The distribution of the single domains of EQ-5D-5L in survivors. Shows the distributions of the single domains of EQ-5D-5L in the two groups among survivors only (n = 512). The proportions of relatives answering the HRQoL questionnaire on behalf of patients was 5 of 277 (1.7%) in the haloperidol group and 6 of 235 (2.6%) in the placebo group. The numeric data corresponding to the figure are presented in Table S2 in the ESM 1
Discussion
In this 1-year follow-up study of the AID-ICU trial we assessed the long-term effects of haloperidol versus placebo on mortality and HRQoL in acutely admitted adult ICU patients with delirium. We found that haloperidol reduced mortality, but did not statistically significantly improve HRQoL, although the HRQoL results had a higher uncertainty.
Mortality
The effect of haloperidol treatment on short-term mortality (28 and 90 day mortality) has been explored in different randomised clinical trials with differing results [16, 36,37,38]. This may be explained by differences in the recruited trial populations (prevention vs. treatment trials) and settings. Differences between the two largest treatment randomised controlled trials (RCTs) has recently been discussed in this journal [39]. The present report is the first on treatment effects of haloperidol on long-term mortality and our results are consistent with a recent systematic review assessing effects of haloperidol versus placebo on mortality and serious adverse events in critically ill patients with delirium. This review concluded that haloperidol may reduce mortality as a meta-analysis of data from five trials and 1553 patients found a relative risk reduction of 0.89 (96.7% CI 0.77–1.03) [11]. This is currently the best estimate for the effect of haloperidol on short-term mortality (i.e., to a maximum of 90 days), which still includes some uncertainty and is primarily driven by data from the AID-ICU trial. In the present study we report the long-term effect on mortality of haloperidol versus placebo, and report an effect size that is in line with the primary publication of the AID-ICU trial [16]. We observed a separation between the survival curves early in the intervention period (day 5–10 post-randomisation) and this separation remained until day 365. The effect estimate for survival was almost constant from 90-day to 1-year follow-up. The early separation of the curves could indicate that haloperidol has an effect on delirium management and that this effect is translated into improved long-term survival.
Differing results concerning mortality has also been reported in observational studies, where several cohort studies have found an association between delirium in the ICU and increased long-term mortality (6 months up to 18 months) [5, 40,41,42], and others (up to 24 months) found no statistically significant associations [43] The data we present here are the first from an RCT.
Health-related quality of life
We provide the first randomised data on the long-term effect of haloperidol on HRQoL in critically ill patients with delirium and found no effect, but some uncertainty remains as 95% CIs were broad and contain potentially clinically important differences; hence, such differences cannot be ruled out. Although no statistically significant impact of haloperidol treatment of HRQoL was found in this study, a positive aspect is the absence of a decline in overall HRQoL despite the increase in survival. Nonetheless, it is important to note that increased survival does not automatically translate into improved HRQoL, meaning that patients who survived do not necessarily experience improved quality of life but may instead survive with more disability and, therefore report worse HRQOL [44].
Several Dutch observational studies using EQ-5D-5L to explore HRQoL have reported an association between ICU delirium and worse long-term HRQoL [45, 46], while other studies found no association [40, 42]. Two of these observational studies reported HRQoL levels, which are in line with the HRQoL levels of this study. However, major differences exist between research design, use of restraints, duration of mechanical ventilation and age, and no studies used EQ VAS, making further comparison difficult [40, 45].
This underlines that future trials should explore patient-important long-term outcomes of delirium, such as HRQoL, cognitive function and mortality [39, 47]. This aligns with the recently published Core Outcome Set for Research Evaluating Interventions to Prevent and/or Treat Delirium in Critically Ill Adults (Del-COrS) [48].
Our trial has several strengths. First, this was a pre-planned 1-year follow-up study with a published protocol [18]. Second, we achieved high data completeness for mortality and only some missingness for HRQoL. Third, data was collected in a large, multicentre RCT and patients, clinical personnel, researchers, and outcome assessors were blinded for the intervention. Fourth, we used a generic instrument for collecting HRQoL which is validated and recommended for follow-up of critically ill patients [48, 49]. These factors may increase the internal validity of our results. Fifth, we pre-specified that deceased patients would be assigned zero for HRQoL measures, supplemented with secondary analyses conducted in survivors only. We expected high mortality in this population and that our intervention could have an effect on mortality; excluding deceased patients from HRQoL could, therefore, be misleading [50, 51]. This choice also aligns with EuroQoL’s recommendations as they define an EQ-5D-5L index score of zero to be equal to death [25]. It is possible for alive patients to have self-reported HRQoL below zero indicated HRQoL worse than being dead [44].
Our study also has limitations. First, for HRQoL the proportion of missing data were above the pre-specified 5% threshold. To mitigate the potential bias of missing data, we performed multiple imputations and best–worst/worst-case analyses according to protocol and recommendations [18, 52]. These analyses found that missing data potentially could affect the results in both directions. Second, most patients (96.4%) were randomised in Danish ICUs, which may limit the external validity of the study. Thirdly, no value set is available for Finland, we therefore used the Danish value set to calculate index values for Finnish patients.
In conclusion, in acutely admitted adult ICU patients with delirium, haloperidol treatment reduced mortality at 1-year follow-up, but did not statistically significantly improve HRQoL.
Data availability
Fully anonymised data are available upon request following approval by the management committee.
References
Association AP (2013) Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Pub
Krewulak KD, Stelfox HT, Leigh JP et al (2018) Incidence and prevalence of delirium subtypes in an adult ICU: a systematic review and meta-analysis. Crit Care Med 46:2029–2035. https://doi.org/10.1097/CCM.0000000000003402
Sachdev PS, Blacker D, Blazer DG et al (2014) Classifying neurocognitive disorders: the DSM-5 approach. Nat Rev Neurol 10:634–642. https://doi.org/10.1038/nrneurol.2014.181
Ankravs MJ, McKenzie CA, Kenes MT (2023) Precision-based approaches to delirium in critical illness: a narrative review. Pharmacotherapy. https://doi.org/10.1002/phar.2807
Pisani MA, Kong SYJ, Kasl SV et al (2009) Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med 180:1092–1097. https://doi.org/10.1164/rccm.200904-0537OC
Pandharipande PP, Girard TD, Jackson JC et al (2013) Long-term cognitive impairment after critical illness. N Engl J Med 369:1306–1316. https://doi.org/10.1056/NEJMoa1301372
Altman MT, Knauert MP, Murphy TE et al (2018) Association of intensive care unit delirium with sleep disturbance and functional disability after critical illness: an observational cohort study. Ann Intensive Care 8:63. https://doi.org/10.1186/s13613-018-0408-4
Devlin JW, Needham DM (2021) Long-term outcomes after delirium in the ICU: addressing gaps in our knowledge. Am J Respir Crit Care Med 204:383–385. https://doi.org/10.1164/rccm.202104-0910ED
Burry L, Mehta S, Perreault MM et al (2018) Antipsychotics for treatment of delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD005594.pub3
Burry L, Hutton B, Williamson DR et al (2019) Pharmacological interventions for the treatment of delirium in critically ill adults. Cochrane Database Syst Rev 2019:CD011749. https://doi.org/10.1002/14651858.CD011749.pub2
Andersen-Ranberg NC, Barbateskovic M, Perner A et al (2023) Haloperidol for the treatment of delirium in critically ill patients: an updated systematic review with meta-analysis and trial sequential analysis. Crit Care 27:329. https://doi.org/10.1186/s13054-023-04621-4
Burry LD, Cheng W, Williamson DR et al (2021) Pharmacological and non-pharmacological interventions to prevent delirium in critically ill patients: a systematic review and network meta-analysis. Intensive Care Med 47:943–960. https://doi.org/10.1007/s00134-021-06490-3
Poulsen LM, Estrup S, Mortensen CB, Andersen-Ranberg NC (2021) Delirium in intensive care. Curr Anesthesiol Rep. https://doi.org/10.1007/s40140-021-00476-z
Collet MO, Caballero J, Sonneville R et al (2018) Prevalence and risk factors related to haloperidol use for delirium in adult intensive care patients: the multinational AID-ICU inception cohort study. Intensive Care Med. https://doi.org/10.1007/s00134-018-5204-y
Barbateskovic M, Krauss SR, Collet MO et al (2019) Haloperidol for the treatment of delirium in critically ill patients: a systematic review with meta-analysis and Trial Sequential Analysis. Acta Anaesthesiol Scand. https://doi.org/10.1111/aas.13501
Andersen-Ranberg NC, Poulsen LM, Perner A et al (2022) Haloperidol for the treatment of delirium in ICU patients. N Engl J Med. https://doi.org/10.1056/NEJMoa2211868
Andersen-Ranberg NC, Poulsen LM, Perner A et al (2023) Haloperidol vs. placebo for the treatment of delirium in ICU patients: a pre-planned, secondary Bayesian analysis of the AID-ICU trial. Intensive Care Med 49:411–420. https://doi.org/10.1007/s00134-023-07024-9
Mortensen CB, Poulsen LM, Andersen-Ranberg NC et al (2020) Mortality and HRQoL in ICU patients with delirium: protocol for 1-year follow-up of AID-ICU trial. Acta Anaesthesiol Scand 64:1519–1525. https://doi.org/10.1111/aas.13679
Andersen-Ranberg NC, Poulsen LM, Perner A et al (2019) Agents intervening against delirium in the intensive care unit (AID-ICU) - Protocol for a randomised placebo-controlled trial of haloperidol in patients with delirium in the ICU. Acta Anaesthesiol Scand 63:1426–1433. https://doi.org/10.1111/aas.13453
World Medical Association (2013) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310:2191–2194. https://doi.org/10.1001/jama.2013.281053
Andersen-Ranberg N, Poulsen LM, Perner A et al (2020) The Agents Intervening against Delirium in the Intensive Care Unit-Trial (AID-ICU trial): a detailed statistical analysis plan. Acta Anaesthesiol Scand. https://doi.org/10.1111/aas.13661
Schulz KF, Altman DG, Moher D, CONSORT Group (2010) CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 340:c332. https://doi.org/10.1136/bmj.c332
Ely EW, Margolin R, Francis J et al (2001) Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med 29:1370–1379
Boettger S, Nuñez DG, Meyer R et al (2017) Delirium in the intensive care setting: a reevaluation of the validity of the CAM–ICU and ICDSC versus the DSM–IV–TR in determining a diagnosis of delirium as part of the daily clinical routine. Palliat Support Care 15:675–683. https://doi.org/10.1017/S1478951516001176
Herdman M, Gudex C, Lloyd A et al (2011) Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 20:1727–1736. https://doi.org/10.1007/s11136-011-9903-x
Euroqol Crosswalk Index Value Calculator – EQ-5D. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/valuation-standard-value-sets/crosswalk-index-value-calculator/. Accessed 1 Mar 2020
Jensen MB, Jensen CE, Gudex C et al (2023) Danish population health measured by the EQ-5D-5L. Scand J Public Health 51:241–249. https://doi.org/10.1177/14034948211058060
Devlin NJ, Shah KK, Feng Y et al (2018) Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 27:7–22. https://doi.org/10.1002/hec.3564
McNamara S, Schneider PP, Love-Koh J et al (2023) Quality-adjusted life expectancy norms for the English population. Value Health 26:163–169. https://doi.org/10.1016/j.jval.2022.07.005
Devlin N, Roudijk B, Ludwig K (2022) Value sets for EQ-5D-5L: a compendium, comparative review & user guide. Springer International Publishing, Cham
Jensen CE, Sørensen SS, Gudex C et al (2021) The Danish EQ-5D-5L value set: a hybrid model using cTTO and DCE data. Appl Health Econ Health Policy 19:579–591. https://doi.org/10.1007/s40258-021-00639-3
Dowdy DW, Eid MP, Sedrakyan A et al (2005) Quality of life in adult survivors of critical illness: a systematic review of the literature. Intensive Care Med 31:611–620. https://doi.org/10.1007/s00134-005-2592-6
Granholm A, Perner A, Krag M et al (2018) Development and internal validation of the simplified mortality score for the intensive care unit (SMS-ICU). Acta Anaesthesiol Scand 62:336–346. https://doi.org/10.1111/aas.13048
Bland JM, Altman DG (2015) Statistics notes: bootstrap resampling methods. BMJ 350:h2622–h2622. https://doi.org/10.1136/bmj.h2622
Jensen AK, Lange T (2019) A novel high-power test for continuous outcomes truncated by death. arXiv: https://arxiv.org/1910.12267 [stat]
Page VJ, Ely EW, Gates S et al (2013) Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 1:515–523. https://doi.org/10.1016/S2213-2600(13)70166-8
Girard TD, Exline MC, Carson SS et al (2018) Haloperidol and ziprasidone for treatment of Delirium in critical illness. N Engl J Med 379:2506–2516. https://doi.org/10.1056/NEJMoa1808217
van den Boogaard M, Slooter AJC, Brüggemann RJM et al (2018) Effect of haloperidol on survival among critically Ill adults with a high risk of delirium: the REDUCE randomized clinical trial. JAMA 319:680–690. https://doi.org/10.1001/jama.2018.0160
Andersen-Ranberg NC, Girard TD, Perner A (2023) Haloperidol and delirium: what is next? Intensive Care Med. https://doi.org/10.1007/s00134-023-07232-3
Wolters AE, van Dijk D, Pasma W et al (2014) Long-term outcome of delirium during intensive care unit stay in survivors of critical illness: a prospective cohort study. Crit Care 18:R125. https://doi.org/10.1186/cc13929
Ely EW, Shintani A, Truman B et al (2004) Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 291:1753–1762. https://doi.org/10.1001/jama.291.14.1753
van den Boogaard M, Schoonhoven L, Evers AWM et al (2012) Delirium in critically ill patients: impact on long-term health-related quality of life and cognitive functioning. Crit Care Med 40:112–118. https://doi.org/10.1097/CCM.0b013e31822e9fc9
Fiest KM, Soo A, Hee Lee C et al (2021) Long-term outcomes in ICU patients with delirium: a population-based cohort study. Am J Respir Crit Care Med 204:412–420. https://doi.org/10.1164/rccm.202002-0320OC
Pallanch O, Ortalda A, Pelosi P et al (2022) Effects on health-related quality of life of interventions affecting survival in critically ill patients: a systematic review. Crit Care 26:126. https://doi.org/10.1186/s13054-022-03993-3
van der Heijden EFM, Kooken RWJ, Zegers M et al (2023) Differences in long-term outcomes between ICU patients with persistent delirium, non-persistent delirium and no delirium: a longitudinal cohort study. J Crit Care 76:154277. https://doi.org/10.1016/j.jcrc.2023.154277
Hofhuis JGM, Schermer T, Spronk PE (2022) Mental health-related quality of life is related to delirium in intensive care patients. Intensive Care Med 48:1197–1205. https://doi.org/10.1007/s00134-022-06841-8
Salluh JIF, Wang H, Schneider EB et al (2015) Outcome of delirium in critically ill patients: systematic review and meta-analysis. BMJ 350:h2538. https://doi.org/10.1136/bmj.h2538
Rose L, Blackwood B, Needham DM et al (2023) Measures for the core outcome set for research evaluating interventions to prevent and/or treat delirium in critically ill adults: an international consensus study (Del-COrS). Crit Care Explor 5:e0884. https://doi.org/10.1097/CCE.0000000000000884
Dinglas VD, Cherukuri SPS, Needham DM (2020) Core outcomes sets for studies evaluating critical illness and patient recovery. Curr Opin Crit Care 26:489–499. https://doi.org/10.1097/MCC.0000000000000750
Colantuoni E, Li X, Hashem MD et al (2021) A structured methodology review showed analyses of functional outcomes are frequently limited to “survivors only” in trials enrolling patients at high risk of death. J Clin Epidemiol 137:126–132. https://doi.org/10.1016/j.jclinepi.2021.03.027
Granholm A, Anthon CT, Kjær M-BN et al (2022) Patient-important outcomes other than mortality in contemporary ICU trials: a scoping review. Crit Care Med. https://doi.org/10.1097/CCM.0000000000005637
Jakobsen JC, Gluud C, Wetterslev J, Winkel P (2017) When and how should multiple imputation be used for handling missing data in randomised clinical trials—a practical guide with flowcharts. BMC Med Res Methodol 17:162. https://doi.org/10.1186/s12874-017-0442-1
Acknowledgements
We thank everyone involved in the AID-ICU trial (patients, relatives, clinicians, research staff, investigators and funding sources).
Funding
Open access funding provided by Lund University. The AID-ICU trial is funded by The Innovation Fund Denmark, Zealand Region Research Fund, Foght Foundation, Hindsgavl Intensive Care Symposium, and the Regions of Denmark Medical Research Fund. The funders had no influence on trial design, conduct, or report. This research was supported by Innovationsfonden (Grant 4108-00011B).
Author information
Authors and Affiliations
Contributions
CBEM wrote the first draft of this manuscript, which all authors critically revised and approved for publication. NCAN was the coordinating investigator of the AID-ICU trial. NCAN was responsible for data management, figures, and tables and the Kaplan-Meyer and COX-regression were performed by CBEM and NCAN and AG performed the analyses.
Corresponding author
Ethics declarations
Conflicts of interest
AG, MBNK, MOC and AP are affiliated with the Department of Intensive Care at Rigshospitalet, which has received funding for other projects from The Novo Nordisk Foundation, Pfizer, and Fresenius Kabi, Sygeforsikringen “danmark”. AP has received an honorarium from Novartis for the participation in an advisory board. AP is on DSMB for The Mega-Rox trial, UK-Rox trial, Bone Zone trial. BSR are affiliated with the Department of Anaesthesia and Intensive Care, Aalborg University Hospital, Aalborg, Denmark, which has received funding for other project from Novo Nordisk Foundation, Danish Ministry of Education and Science and Beckett Foundation. MHB are affiliated with the Department of Anaesthesia and Intensive Care at Copenhagen University Hospital – North Zealand, which has received funding for other research projects from The Novo Nordisk Foundation, Sygeforsikringen “danmark”, Toyota Foundation, A.P. Moeller Foundation, Frimodt-Heineke Foundation, Svend Andersen Foundation, Ehrenreich Foundation, and Olga Bryde Nielsen Foundation, and has conducted contract research for AM-Pharma (the REVIVAL trial) and Inotrem (ASTONISH trial). MHB has received an honorarium from AM-Pharma for participation in an advisory board. TL is a member of DSMB for Novo Nordisk and Leo Pharma study. BE is part of the Advisory Board of Eli Lilly Denmark A/S, Janssen-Cilag, Lundbeck Pharma A/S, and Takeda Pharmaceutical Company Ltd; and has received lecture fees from Bristol-Myers Squibb, Boehringer Ingelheim, Otsuka Pharma Scandinavia AB, Eli Lilly Company, and Lundbeck Pharma A/S. JH has received funding from Paulo foundation, Finska Läkaresällskapet, NordForsk and Tor och Kirsti Johanssons Hjärt och Cancerstiftelse. GC has received istituational research grants from Neuroptics and Integra and is a board member at Neuroptics, Integra, Biogen, Invex Therapeutics and Idorsia. All other authors have no conflicts to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Mortensen, C.B., Andersen-Ranberg, N.C., Poulsen, L.M. et al. Long-term outcomes with haloperidol versus placebo in acutely admitted adult ICU patients with delirium. Intensive Care Med 50, 103–113 (2024). https://doi.org/10.1007/s00134-023-07282-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-023-07282-7