Abstract
Purpose
The Acute Disease Quality Initiative (ADQI) Workgroup recently released a consensus definition of sepsis-associated acute kidney injury (SA-AKI), combining Sepsis-3 and Kidney Disease Improving Global Outcomes (KDIGO) AKI criteria. This study aims to describe the epidemiology of SA-AKI.
Methods
This is a retrospective cohort study carried out in 12 intensive care units (ICUs) from 2015 to 2021. We studied the incidence, patient characteristics, timing, trajectory, treatment, and associated outcomes of SA-AKI based on the ADQI definition.
Results
Out of 84,528 admissions, 13,451 met the SA-AKI criteria with its incidence peaking at 18% in 2021. SA-AKI patients were typically admitted from home via the emergency department (ED) with a median time to SA-AKI diagnosis of 1 day (interquartile range (IQR) 1–1) from ICU admission. At diagnosis, most SA-AKI patients (54%) had a stage 1 AKI, mostly due to the low urinary output (UO) criterion only (65%). Compared to diagnosis by creatinine alone, or by both UO and creatinine criteria, patients diagnosed by UO alone had lower renal replacement therapy (RRT) requirements (2.8% vs 18% vs 50%; p < 0.001), which was consistent across all stages of AKI. SA-AKI hospital mortality was 18% and SA-AKI was independently associated with increased mortality. In SA-AKI, diagnosis by low UO only, compared to creatinine alone or to both UO and creatinine criteria, carried an odds ratio of 0.34 (95% confidence interval (CI) 0.32–0.36) for mortality.
Conclusion
SA-AKI occurs in 1 in 6 ICU patients, is diagnosed on day 1 and carries significant morbidity and mortality risk with patients mostly admitted from home via the ED. However, most SA-AKI is stage 1 and mostly due to low UO, which carries much lower risk than diagnosis by other criteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Sepsis-associated acute kidney injury (SA-AKI) is a common, increasingly prevalent problem in the intensive care unit (ICU). It occurs predominantly in patients admitted from the emergency department and is usually diagnosed within a day of ICU admission. Most patients with SA-AKI had stage 1 AKI and were diagnosed by low urine output alone, a group in which deterioration in renal function was uncommon. |
Introduction
Sepsis is a common cause of critical illness and is associated with high morbidity and mortality [1,2,3] and, often, with acute kidney injury (AKI). When AKI occurs in this setting, it is referred to as sepsis-associated acute kidney injury (SA-AKI) [4, 5]. The association between sepsis and AKI has been studied previously [6, 7]. However, the lack of a reproducible and standardized consensus definition has limited the interpretability of available knowledge.
A definition of SA-AKI was recently produced by the Acute Disease Quality Initiative (ADQI) 28 Workgroup [8]. It combines the presence of sepsis, defined by the Sepsis-3 criteria [9], with the presence of AKI, defined by the Kidney Disease Improving Global Outcomes (KDIGO) criteria [10], occurring within 7 days of the diagnosis of sepsis.
The epidemiology of SA-AKI in the critically ill, based on this standardised consensus definition, remains unknown. Furthermore, its incidence, patient characteristics, timing, trajectory, treatment, and associated outcomes have not been studied. Guided by the ADQI 28 Workgroup’s consensus definition and research priorities, we analyzed a large, granular, multicentre database of routinely collected electronic medical record (EMR) data to assess SA-AKI.
We aimed to test the primary hypothesis that SA-AKI is common in patients admitted to the intensive care unit (ICU). We also intended to test the secondary hypothesis that most SA-AKI is not a disease of intensive care, but rather a disease that develops outside intensive care and triggers intensive care admission. We also tested the secondary hypothesis that patients with SA-AKI had worse renal and non-renal outcomes than non-SA-AKI patients.
Methods
Study design
Large, multicentre, retrospective cohort study of granular, routinely collected, EMR-based clinical data.
Study sites
The study sites were 12 closed-model ICUs located in Queensland, Australia. The ICUs included 5 tertiary ICUs, 3 outer metropolitan ICUs, and 4 regional ICUs. The centres comprise most of the entire state-wide ICU capacity and include all the state-wide referral centres for cardiothoracic, neurosurgical, obstetric, and trauma patients, as well as outer metropolitan and regional ICUs. We evaluated all adult patients admitted between January 1st, 2015, and December 31st, 2021. Of note, Queensland, Australia did not have a community spread of coronavirus disease 2019 (COVID-19) during the study dates, therefore, the global pandemic did not have a significant impact on the study. All patients were eligible if their electronic medical records were retrievable. We excluded patients with advanced chronic kidney disease requiring chronic dialysis, patients admitted with palliative intent, and patients transferred from another participating ICU. We did not exclude readmission episodes within the same hospital admission.
Data sources
Routinely collected data were obtained from all centres using the eCritical MetaVision™ (iMDsoft, Boston, MA, USA) clinical information systems. This included daily laboratory data, daily medications, daily microbiology, as well as hourly haemodynamic and hourly fluid balance data. Information on baseline demographics, admission diagnosis, the severity of illness and outcomes were extracted from the Australia and Zealand Intensive Care Society (ANZICS) Centre for Outcome and Resource Evaluation (CORE) Adult Patient Database (APD) [11,12,13,14]. The amount of missing data for key variables is shown in Supplementary Table S1.
Identification of sepsis
Sepsis was defined according to the third international consensus definitions for sepsis and septic shock [9]. According to the SEPSIS-3 definition, we identified patients with an increase in Sequential Organ Failure Assessment (SOFA) score by two points with proven or suspected infection [9]. We assumed a SOFA score of zero before ICU admission. Where individual components of SOFA were missing, no contribution was made to the total score [12]. The daily total SOFA score was calculated and an increase of two points over 24 hours was identified. Given the challenges of interpreting neurological SOFA with concurrent sedation, it was not included in the total SOFA [15]. Proven or suspected infection was defined as the commencement or escalation of antimicrobial therapy and microbiological sampling within 1 day of the SOFA score increase [16]. An escalation of antimicrobials was defined as an increase in antimicrobial ‘rank’ within 1 day of diagnosis of sepsis [17, 18]. As demonstrated in Supplementary Table S2, ranking corresponds to the spectrum of activity with rank one indicating the lowest spectrum, such as first-generation cephalosporin, and rank four the highest spectrum, such as carbapenems or tigecycline [18].
Patients admitted post-elective surgery, trauma, or cardiac arrest were assumed to be receiving antibiotic prophylaxis in the first 2 days of admission. Thus, they were not classified as having sepsis regardless of their SOFA score. Septic shock was defined as the administration of a vasopressor medication and at least one blood lactate greater than 2 mmol/L on the day of sepsis diagnosis [19]. Furthermore, the dosage of vasopressor was converted to norepinephrine equivalent, with conversion method shown in Supplementary Table S3 [20].
Identification of acute kidney injury
AKI was defined according to the KDIGO definition, with daily serum creatinine and hourly urine output (UO) data [10]. To manage absent hourly UO measurements secondary to the absence of an indwelling catheter or transfer outside of ICU, we performed imputation as described in the Supplementary Table S4. Hourly UO per kilogram of body weight was assessed on a rolling basis to identify patients who met AKI UO criteria. Every sliding 6, 12, and 24-h window of UO was assessed against AKI thresholds. We performed multiple sensitivity analyses assessing for systematic differences based on UO imputation (Supplementary Tables S5, S6). Our dataset did not have pre-ICU creatinine data, therefore the baseline creatinine was estimated using the Chronic Kidney Disease–Epidemiology Collaboration (CKD-EPI) equation assuming an estimated glomerular filtration rate (eGFR) of 75 mL/min/1.73m2 [21, 22]. Daily serum creatinine was compared to estimated baseline creatinine and values meeting definition of AKI were recorded. Multiple sensitivity analyses comparing different methods to estimated baseline serum creatinine were performed (Supplementary Table S7). Urine output-based or creatinine-based criteria or both were used to identify whether a patient met the KDIGO criteria for AKI.
Identification of sepsis-associated acute kidney injury
After individually identifying episodes of sepsis and AKI, we applied the ADQI 28 Workgroup SA-AKI definition. We compared the day of the sepsis diagnosis to the day of the AKI diagnosis. If AKI occurred between days 1 and 7 after sepsis diagnosis, patients were classified as SA-AKI according to the ADQI criteria. Patients did not meet the definition of SA-AKI, if AKI preceeded episode of sepsis. A sensitivity analysis comparing patients diagnosed with sepsis or AKI, but not meeting SA-AKI criteria was performed (Supplementary Fig. S8).
Outcomes
The primary outcome was the incidence of SA-AKI. The secondary outcome was the timing of SA-AKI in relation to ICU admission. The additional secondary outcomes are renal, AKI severity, renal recovery status, and major adverse kidney events at 30 days (MAKE-30), and non-renal, ICU and hospital length of stay, and ICU and hospital mortality. Outcome definitions are provided in Supplementary Fig. S11.
Statistical analysis
Descriptive statistics were expressed as frequencies and proportions for categorical variables and medians with interquartile ranges (IQR) or means with standard deviations depending on their parametric or non-parametric distribution. Fisher’s exact test was used to compare categorical data. A mixed-effect logistic regression model, including hospitals as a random effect, was developed to examine factors associated with hospital mortality and major adverse kidney events at day 30 (MAKE-30). The variables used for analysis were determined a priori and reflected the clinical utility of available data. The results of the multivariable analysis were reported as odds ratios (OR) with 95% confidence intervals (95% CI). Given the large data set a two-sided p value of < 0.01 was considered statistically significant. Statistical analyses were performed using R v.4.0.3.
Ethical considerations
This study was approved by the Metro South Hospital and Health Service Human Research Ethics Committee (HREC/2022/QMS/82024) with an individual waiver of consent granted.
Results
Incidence of SA-AKI
From January 1st, 2015, to December 31st, 2021, 89,466 patients were admitted to the participating ICUs. We excluded 1950 patients with end-stage kidney disease, 544 patients admitted with palliative intent, and 2,503 patients transferred between facilities. Of the remaining 84,528 patients, 13,451 met the criteria for SA-AKI during their ICU admission. As demonstrated in Supplementary Fig. S1, the percentage of admissions that met SA-AKI criteria increased over time from 14% in 2015 to nearly 18% in 2021.
Patient characteristics
The overall patient characteristics, inclusive of both non-SA-AKI and SA-AKI patients, are shown in Table 1. In the entire cohort, the median age was 61 years, 39% were female, and the median body mass index (BMI) was 27.8. The severity of illness as measured by the median Acute Physiology and Chronic Health Evaluation (APACHE) III score was 50 and the most common source of admission was the operating room followed by the emergency department.
When compared to the entire ICU cohort, SA-AKI patients had a higher APACHE III score, were more likely to be admitted from the emergency department or a hospital ward and had a higher risk of death. Though statistically different, the SA-AKI group had a numerically similar age, sex distribution, and body mass index to the entire cohort.
On the day of ICU admission, most SA-AKI patients required ventilation, which was similar to non-SA-AKI patients. Almost half received at least 1 hour of vasopressor therapy and almost one in ten required renal replacement theraphy (RRT), all of which were more common than in non-SA-AKI patients. Of note, diuretic therapy was rarely used.
Timing and characteristics of SA-AKI diagnosis
As shown in Table 2, the median day of sepsis diagnosis was the day of ICU admission, or day 1 (IQR 1–1). At the time of SA-AKI diagnosis, over a third (40%) of patients had septic shock and the median SOFA score at sepsis diagnosis of 7 (IQR 5–9). Most patients met AKI criteria on the same day as sepsis criteria, with a median time from sepsis to AKI of 0 days (IQR 0–1). Most patients (44%), were diagnosed with AKI based on UO alone, whereas 35% and 21% met creatinine criteria alone or both UO and creatinine criteria, respectively. At AKI diagnosis, the majority (54%) had stage 1 AKI, whereas only 25% and 21% had stage 2 and stage 3 AKI, respectively. Moreover, 11% of patients received RRT on the day of SA-AKI diagnosis. As demonstrated in Supplementary Table S4, 65% (5,700) of SA-AKI patients with stage 1 AKI were diagnosed by UO alone, representing 35% of the entire SA-AKI cohort.
Trajectory of SA-AKI
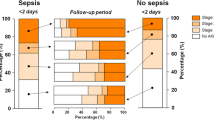
We examined the trajectory of SA-AKI patients by daily serum creatinine and hourly UO over 14 days. The mean serum creatinine of all SA-AKI patients stabilised at day six of admission at a mean of 130 µmol/L from a peak of > 170 µmol/L in survivors and at a mean of 155 µmol/L from a peak of 180 µmol/L in patients who died in hospital (Supplementary Fig. S2). Patients with stage 1 and stage 2 AKI did not have major fluctuations in mean serum creatinine over time. The mean serum creatinine of patients with Stage 3 AKI declined from a peak of almost 400 µmol/L on day one then remained level from day 6 onwards at 200 µmol/L (Supplementary Fig. S3). As most patients were diagnosed with AKI by UO alone, we examined serum creatinine over time by AKI diagnostic criteria. In patients diagnosed by UO alone, the mean serum creatinine remained unchanged and within normal range throughout the ICU admission (Fig. 1). These findings were consistent across each stage of AKI (Figs. S4, S6). Furthermore, among patients diagnosed by UO alone, the hourly UO data for all AKI stages demonstrated that normalisation of UO was typical and occurred within 24–48 hours of ICU admission (Supplementary Figs. S7, S9).
In contrast, patients diagnosed with AKI by both UO and creatinine had a persistently lower UO, an elevated serum creatinine, the mean hourly UO never increased above 1 mL/kg/h (Supplementary Fig. S10), and the serum creatinine remained elevated at ≥ 200 µmol/L (Fig. 1).
A summary of the trajectory of AKI stages over time for the entire SA-AKI cohort is shown in Supplementary Fig. S11, demonstrating that stage 1 or stage 2 AKI patients decreased in number with resolution of AKI, whereas, many stage 3 AKI patients maintained their stage throughout their ICU admission. The trajectory of SA-AKI patients diagnosed with AKI by UO alone is shown in Supplementary Fig. S12, demonstrating rapid resolution of AKI in the majority of patients.
Treatment during first 7 days of ICU admission
ICU therapies were examined during the first 7 days of ICU admission, a duration which represents the top quartile of ICU length of stay. Compared to patients diagnosed by creatinine alone or both UO and creatinine, patients who were diagnosed with SA-AKI by UO alone were more likely to receive ventilation, but were significantly less likely to require vasopressors and RRT (Table 3). Except for ventilation in stage 3 AKI, these findings were consistent across all stages of AKI severity (Supplementary Tables S11–S13).
In SA-AKI patients, when compared to stage 1 and stage 2, patients with stage 3 were less likely to receive ventilation, received fewer days of vasopressor, and were more likely to require RRT (Supplementary Table S14), which may represent a possible interaction with chronic kidney disease (CKD), which was not available in the data. For the entire cohort, patients with SA-AKI were more likely to require ventilation, vasopressors, and RRT (Supplementary Table S15).
Associated outcomes
As shown in Table 4, we examined outcomes based on the AKI diagnostic criteria met (UO alone, creatinine alone, or both). Patients diagnosed with AKI only due to low UO, had lower severity of maximum AKI, less need for RRT, higher rate of renal recovery, and lower mortality, when compared to diagnosis with both, or serum creatinine alone. To further determine the impact of severity of AKI at diagnosis, we compared outcomes for AKI diagnostic criteria met in each AKI severity group. In stage 1 AKI, when compared to creatinine alone or both, low UO consistently had lower severity of AKI, a much lower RRT requirement, lower mortality, and a MAKE-30 demonstrating few adverse renal outcomes (Supplementary Table S16). The relatively low requirement for RRT, low mortality and almost absence of adverse renal outcomes in patients diagnosed by UO alone was consistent in stage 2 and stage 3 AKI (Supplementary Tables S17, S18). In all stages of AKI, patients diagnosed by UO only had a three times higher chance of complete renal recovery, compared to those diagnosed by creatinine alone or both UO and creatinine. Compared to the entire cohort, patients with SA-AKI had a longer ICU and hospital length of stay, as well as a higher ICU and hospital mortality (Supplementary Table S19).
For patients with SA-AKI, we created a multivariable logistic regression model for MAKE-30 (Supplementary Table S20). When controlling for patient characteristics, age, sex, BMI, and severity of illness, APACHE 3 score, ventilation requirement, and vasopressor requirement, an AKI diagnosis by UO criteria alone (OR 0.34; 95% CI 0.32–0.36; p < 0.001) was associated with a significant decrease in MAKE-30 when compared to creatinine alone and both criteria.
Discussion
Key findings
In this multicentre study, we investigated close to 90,000 critically ill patients and found that SA-AKI occurred in one-sixth of all ICU admissions, that four out of ten such patients were emergency admissions from the community and one in four from the hospital wards. Furthermore, the proportion of ICU admissions diagnosed with SA-AKI has increased year on year. When compared to other ICU admissions, patients with SA-AKI had a higher severity of illness and received more organ support therapies.
SA-AKI predominantly occurred within 24 hours of ICU admission and the diagnosis of sepsis and AKI were essentially simultaneous. At diagnosis, however, most patients had stage 1 AKI due to low UO and relatively low serum creatinine. This cohort typically had early normalisation of UO with the resolution of AKI. Overall, patients with SA-AKI who met AKI diagnostic criteria by low UO alone had a shorter length of stay in the ICU and hospital, as well as lower mortality compared to the remainder of the SA-AKI patients, Finally, when adjusting for baseline characteristics and severity of illness, SA-AKI by UO alone had significantly less MAKE-30 events.
Relationship to literature
To our knowledge, no other study has examined the epidemiology of SA-AKI in a large multicenter cohort of patients admitted to ICU utilising the recent consensus ADQI definition. Before such a definition, previous research demonstrated significant heterogeneity in patient populations, with varying incidence and mortality [8]. Two previously published studies, however, used the SEPSIS-3 and KDIGO criteria to define SA-AKI [23, 24]. One was a single-centre retrospective study, only included 351 SA-AKI patients, and reported a very low incidence of SA-AKI at 2.8%, limiting its external validity [23]. Another was a single-centre retrospective study of the association between obesity and AKI in a cohort of 456 patients and did not focus on the epidemiology of SA-AKI [24]. Neither study examined the incidence, characteristics, timing, trajectory, treatment, and outcomes of SA-AKI in detail and a multicentric setting involving a large population. No previous research has assessed the relationship between AKI diagnostic criteria and outcomes in SA-AKI patients.
Implications of the study findings
Our findings imply that SA-AKI is a common and increasingly prevalent condition in the ICU. Furthermore, they suggest that SA-AKI mostly occurs in patients admitted from the community who already present to the ICU with a combination of sepsis and AKI. In another significant proportion, however, it develops in the hospital wards and, as such, it may be a target for earlier intervention.
Furthermore, the rapid resolution, and rare deterioration, of renal function in SA-AKI patients diagnosed by UO alone suggests that, in over half of such patients, the presence of SA-AKI is of limited clinical importance. A finding highlighted by the high prevalence of renal recovery in SA-AKI patients diagnosed with UO alone. Thus, low UO may represent a physiological response to sepsis more than a pathophysiological marker of incipient organ failure. These findings may also have significant future therapeutic and research implications as the current standard of care may be sufficient to manage most cases of stage 1 SA-AKI and prevent adverse outcomes. Moreover, future trials of SA-AKI therapies are unlikely to have sufficient power to detect effects on RRT, MAKE-30, or mortality, if low UO alone SA-AKI patients are included.
Strengths and limitations
Our study had several strengths. First, it was conducted on a large cohort with a wide array of ICU admissions from a state-wide ICU system encompassing a complete range of adult critical care. This population is representative of the general Australian population and likely representative of similar populations in resource-rich countries. Second, our study data were comprehensive with highly granular data, which was electronically extracted from a ubiquitous clinical information system. All data collected were clinically validated and had minimal missing data points. Moreover, given their collection by non-research staff, they represent an unbiased sample. Third, although we utilised a novel standardised definition for SA-AKI, the definition is composed of the SEPSIS-3 and KDIGO AKI definitions, which are well established in the literature. As such, we were able to leverage well-recognised techniques to analyse a large database to identify sepsis and AKI.
We acknowledge some limitations. First, the detection of sepsis and AKI, and therefore, SA-AKI, was done electronically and patients may have been misclassified. Organ dysfunction detected by the analysis may not have been caused by infection. However, our very large cohort of patients likely limits the impact of these unusual circumstances. Second, we did not classify SA-AKI into phenotypes as suggested by the ADQI 28 Workgroup [8]. Thus, our cohort of SA-AKI patients may represent different groups with diverging risk factors and outcomes. We plan to focus future work on such phenotypes. Third, as we could not identify which patients had CKD before presentation, such patients would have led to an overestimation of AKI when using estimated baseline creatinine calculation [25]. This matters, because CKD patients have specific features and different outcomes from those without CKD [26]. However, a previous study in investigated over 3500 of the same patients from four hospitals involved in this study, and using different data reported a low CKD prevalence of 13.5% [27]. Investigation of CKD patients will require additional data acquisition in future studies. Fourth, the censoring of an RRT and serum creatinine components of MAKE-30 at discharge from the ICU potentially introduced ascertainment bias. Lastly, the imputation of hourly UO data could potentially misclassify patients with AKI. However, the sensitivity analysis demonstrates no systemic differences between groups and reduction, not increase, in AKI diagnoses with imputation.
Conclusion
In a large cohort of critically ill patients, we found that SA-AKI is common in patients admitted to the ICU from the ED, mainly occurs within the day of ICU admission, and with sepsis and AKI simultaneously present. Furthermore, most patients with SA-AKI were diagnosed with stage 1 AKI and by low UO alone, which was associated with infrequent deterioration in renal function, as defined by MAKE-30. These observations provide the necessary epidemiological basis for interventional trials, whilst highlighting the need to focus on specific subsets of all SA-AKI patients.
Data availability
Data cannot be shared publicly due to institutional ethics, privacy, and confidentiality regulations. Data released for the purposes of research under section 280 of the Public Health Act 2005 requires an application to the Director-General of Queensland Health (PHA@health.qld.gov.au).
References
Finfer S, Machado FR (2016) The global epidemiology of sepsis. Does it matter that we know so little? Am J Resp Crit Care 193(3):228–230
Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H et al (2006) Sepsis in European intensive care units and colon; results of the SOAP study and ast. Crit Care Med 34(2):344–353
Chiu C, Legrand M (2021) Epidemiology of sepsis and septic shock. Curr Opin Anaesthesiol 34(2):71–76
Poston JT, Koyner JL (2019) Sepsis associated acute kidney injury. BMJ 364:k4891
Peerapornratana S, Manrique-Caballero CL, Gómez H, Kellum JA (2019) Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int 96(5):1083–1099
Bagshaw SM, George C, Bellomo R, Committee ADM (2008) Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care 12(2):R47
Bagshaw SM, Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M et al (2007) Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephro 2(3):431–439
Zarbock A, Nadim MK, Pickkers P, Gomez H, Bell S, Joannidis M et al (2023) Sepsis-associated acute kidney injury: consensus report of the 28th Acute Disease Quality Initiative workgroup. Nat Rev Nephrol. https://doi.org/10.1038/s41581-023-00683-3
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M et al (2016) The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315(8):801–810
Khwaja A (2012) KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 120(4):c179–c184
Bagshaw SM, George C, Bellomo R, Committe ADM (2008) A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transpl 23(5):1569–1574
Raith EP, Udy AA, Bailey M, McGloughlin S, MacIsaac C, Bellomo R et al (2017) Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA 317(3):290–300
Corrigan C, Duke G, Millar J, Paul E, Butt W, Gordon M et al (2022) Admissions of children and adolescents with deliberate self-harm to intensive care during the SARS-CoV-2 outbreak in australia. Jama Netw Open 5(5):e2211692
Kirsi-Maija K, Michael B, David P, Jamie CD, Rinaldo B (2015) Systemic inflammatory response syndrome criteria in defining severe sepsis. New Engl J Med 372(17):1629–1638
Lambden S, Laterre PF, Levy MM, Francois B (2019) The SOFA score—development, utility and challenges of accurate assessment in clinical trials. Crit Care 23(1):374
Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A et al (2016) Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315(8):762–774
Shah AD, MacCallum NS, Harris S, Brealey DA, Palmer E, Hetherington J et al (2021) Descriptors of sepsis using the sepsis-3 criteria: a cohort study in critical care units within the UK National Institute for Health Research critical care health informatics collaborative. Crit Care Med 49(11):1883–1894
Braykov NP, Morgan DJ, Schweizer ML, Uslan DZ, Kelesidis T, Weisenberg SA et al (2014) Assessment of empirical antibiotic therapy optimisation in six hospitals: an observational cohort study. Lancet Infect Dis 14(12):1220–1227
Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS et al (2016) Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315(8):775–787
Khanna A, English SW, Wang XS, Ham K, Tumlin J, Szerlip H et al (2017) Angiotensin II for the treatment of vasodilatory shock. New Engl J Medicine 377(5):419–430
Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y et al (2021) New creatinine- and cystatin C-based equations to estimate GFR without race. New Engl J Med 385(19):1737–1749
Cooper DJ, Plewes K, Grigg MJ, Patel A, Rajahram GS, William T et al (2021) An evaluation of commonly used surrogate baseline creatinine values to classify AKI during acute infection. Kidney Int Rep 6(3):645–656
Katayama S, Nunomiya S, Koyama K, Wada M, Koinuma T, Goto Y et al (2017) Markers of acute kidney injury in patients with sepsis: the role of soluble thrombomodulin. Crit Care 21(1):229
Gameiro J, Gonçalves M, Pereira M, Rodrigues N, Godinho I, Neves M et al (2018) Obesity, acute kidney injury and mortality in patients with sepsis: a cohort analysis. Ren Fail 40(1):120–126
Bernier-Jean A, Beaubien-Souligny W, Goupil R, Madore F, Paquette F, Troyanov S et al (2017) Diagnosis and outcomes of acute kidney injury using surrogate and imputation methods for missing preadmission creatinine values. Bmc Nephrol 18(1):141
Bagshaw SM, Neto AS, Smith O, Weir M, Qiu H, Du B et al (2022) Impact of renal-replacement therapy strategies on outcomes for patients with chronic kidney disease: a secondary analysis of the STARRT-AKI trial. Intensive Care Med 48:1736
White K, Tabah A, Ramanan M, Shekar K, Edwards F, Laupland KB (2023) 90-day case-fatality in critically ill patients with chronic liver disease influenced by presence of portal hypertension, results from a multicentre retrospective cohort study. J Crit Care 1(38):5–10
Acknowledgements
Queensland Critical Care Research Network Group members: Caboolture Hospital: Mahesh Ramanan, Prashanti Marella and Patrick Young. Cairns Hospital: Pip McIlroy, Ben Nash. Gold Coast University Hospital: James McCullough, Mandy Tallott, Andrea Marshall, David Moore. Logan Hospital: Hayden White, Sunil Sane, Lynette Morrison, Pam Dipplesman. Mackay Hospital: Stephen Luke, Anni Paasilahti, Ray Asimus, Jennifer Taylor, Princess Alexandra Hospital: Kyle White, David Cook, Peter Kruger, Jason Meyer, Rod Hurford. Queensland Children’s Hospital: Kevin Plumpton, Andrew Barlow. Redcliffe Hospital: Alexis Tabah, Hamish Pollock, Patrick Young. Rockhampton Hospital; Antony G Attokaran, Jacobus Poggenpoel, Josephine Reoch. Royal Brisbane and Women’s Hospital: Kevin B Laupland, Felicity Edwards, Jayesh Dhanani, Marianne Kirrane, Pierre Clement, Nermin Karamujic. Sunshine Coast University Hospital: Paula Lister, Vikram Masurkar, Lauren Murray, Jane Brailsford, Todd Erbacher. The Prince Charles Hospital: Kiran Shekar, Jayshree Lavana, George Cornell. Townsville University Hospital: Siva Senthuran, Stephen Whebell, Gail Henson. Queensland University of Technology (QUT): Michelle Gatton, Zephanie Tyack, Robert Andrews, Arthur ter Hofstede, Moe Wynn, Kevin B Laupland, Felicity Edwards.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Consortia
Contributions
The study conception and design (all authors); data acquisition (KW); analysis (AS, KW); interpretation of data (all authors); article drafting (KW), article revision for important intellectual content (all authors); final approval of the version submitted for publication (all authors); agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved (KW, RB).
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Statement of ethics
This study was approved by the Metro South Hospital and Health Service Human Research Ethics Committee (HREC/2022/QMS/82024) with an individual waiver of consent granted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The members of QCCRN Group details are listed in the Acknowledgements.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
White, K.C., Serpa-Neto, A., Hurford, R. et al. Sepsis-associated acute kidney injury in the intensive care unit: incidence, patient characteristics, timing, trajectory, treatment, and associated outcomes. A multicenter, observational study. Intensive Care Med 49, 1079–1089 (2023). https://doi.org/10.1007/s00134-023-07138-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-023-07138-0