Abstract
Purpose
Severe community-acquired pneumonia (CAP) requiring intensive care unit admission is associated with significant acute and long-term morbidity and mortality. We hypothesized that downregulation of systemic and pulmonary inflammation with prolonged low-dose methylprednisolone treatment would accelerate pneumonia resolution and improve clinical outcomes.
Methods
This double-blind, randomized, placebo-controlled clinical trial recruited adult patients within 72–96 h of hospital presentation. Patients were randomized in 1:1 ratio; an intravenous 40 mg loading bolus was followed by 40 mg/day through day 7 and progressive tapering during the 20-day treatment course. Randomization was stratified by site and need for mechanical ventilation (MV) at the time of randomization. Outcomes included a primary endpoint of 60-day all-cause mortality and secondary endpoints of morbidity and mortality up to 1 year of follow-up.
Results
Between January 2012 and April 2016, 586 patients from 42 Veterans Affairs Medical Centers were randomized, short of the 1420 target sample size because of low recruitment. 584 patients were included in the analysis. There was no significant difference in 60-day mortality between the methylprednisolone and placebo arms (16% vs. 18%; adjusted odds ratio 0.90, 95% CI 0.57–1.40). There were no significant differences in secondary outcomes or complications.
Conclusions
In patients with severe CAP, prolonged low-dose methylprednisolone treatment did not significantly reduce 60-day mortality. Treatment was not associated with increased complications.
Similar content being viewed by others
In this double-blind, randomized, placebo-controlled clinical trial of 584 participants hospitalized with severe community-acquired pneumonia, prolonged methylprednisolone treatment did not significantly reduce 60-day all-cause mortality or improve secondary outcomes during initial hospitalization or up to 1 year of follow-up. The risk for complications was similar to the control group. |
Introduction
Pneumonia is the leading cause of community-acquired infection requiring intensive care unit (ICU) admission and a common precipitant of septic shock and acute respiratory distress syndrome (ARDS) [1]. Hospital mortality is higher for patients who are older, bacteremic [2], have more comorbidities [3], meet criteria for healthcare-associated pneumonia (HCAP), require mechanical ventilation (MV) or vasopressor support, or are transferred to the ICU from a medical ward [4]. Most hospital deaths occur after eradication of bacteria from tracheal secretions and the bloodstream [5, 6], implying that adequate antibiotic treatment alone may be insufficient in further improving outcomes. Importantly, patients surviving hospitalization remain at risk for long-term morbidity [7], re-hospitalizations [4], and increased post-discharge mortality at 1 year (21–40%) [4] and up to 5 years [8]. Evidence points to the host’s inability to fully down-regulate systemic inflammation and restore tissue homeostasis as the dominant pathophysiologic processes contributing to acute and chronic adverse outcomes in community-acquired pneumonia (CAP) [9, 10].
Glucocorticoids were investigated in multiple randomized trials, with a signal for benefit in patients with severe pneumonia [11, 12]; however, a large confirmatory study was lacking. The Department of Veterans Affairs (VA) Cooperative Study #574 evaluated the efficacy of prolonged methylprednisolone treatment on short- and long-term morbidity and mortality in patients admitted to the ICU with severe CAP. We hypothesized that a 20-day low-dose methylprednisolone treatment would reduce 60-day mortality and improve clinical outcomes. The rationale for a 20-day treatment was to support the resolution phase of the disease [13], incorporate adequate glucocorticoid tapering [14], and to reduce post-hospitalization low-grade systemic inflammation.
Methods
Trial design and oversight
A double-blind, randomized, placebo-controlled trial was conducted at 42 VA Medical Centers from January 1, 2012 to August 31, 2016. Eligible patients were randomly assigned in a 1:1 ratio to either methylprednisolone or placebo. The trial protocol and the statistical analysis plan are provided in the Supplement Appendix.
The trial was approved by the VA Central Institutional Review Board and conducted in accordance with Good Clinical Practice Guidelines. An independent Data Monitoring Committee monitored patient safety, study conduct, and data. The authors vouch for the accuracy and completeness of the data and statistical analyses and for the fidelity of the trial to the protocol.
Participants
Adult patients presenting with a clinical diagnosis of severe CAP/HCAP were enrolled within 72–96 h (additional 24 h in patients not yet meeting severity criteria) of hospital presentation. Inclusion criteria required the presence of one major or three minor modified American Thoracic Society/Infectious Diseases Society of America (ATS/IDSA) criteria for severe pneumonia [15] as well as admission to intensive or intermediate care. Eligibility criteria are detailed in the Trial Protocol (Supplement Appendix).
Treatment and other trial procedures
Written informed consent was obtained from each participant or their legally authorized representative if they were unable to provide consent. Participants were randomly assigned in a 1:1 ratio to receive methylprednisolone or placebo using random permuted blocks of sizes 2 and 4, stratified by study site and need for MV at enrollment.
Methylprednisolone or placebo was given in a double-blind fashion. On the day of randomization (day 0), an intravenous loading dose of 40 mg was given, followed by maintenance infusion. The full 20-day treatment course included 40 mg/day on days 1–7, 20 mg/day on days 8–14, 12 mg/day on days 15–17 and 4 mg/day on days 18–20. Study drug was given by continuous infusion during ICU stay and changed to twice per day, via intravenous or enteral administration, after ICU discharge. Participants in both groups received standardized care following consensus recommendations [15, 16].
Participants were assessed daily up to day 8 during the initial ICU stay, at hospital discharge, and on days 28, 60, and 180. The final 1-year follow-up for mortality and re-hospitalizations was performed through review of records. We attempted to assess all participants regardless of treatment continuation. Monitoring for serious adverse events (SAEs) continued until the final follow-up contact. Safety monitoring and reporting procedures are detailed in the Trial Protocol.
Outcomes
The primary outcome was all-cause mortality at 60 days. Secondary outcomes included: (1) During hospitalization: post-randomization development of vasopressor-dependent shock or ARDS; number of multiple organ dysfunction syndrome (MODS)-free days to day 8; MV-free days up to days 8 and 28; duration of ICU and hospital stay; potential complications associated with methylprednisolone treatment; and hospital mortality; (2) Post-discharge: cardiovascular complications within 180 days of randomization; quality of life and functional status at days 28, 60, and 180; number and causes of re-hospitalization at VA hospitals within 1 year; SAEs and complications; and all-cause mortality at days 180 and 365. Exploratory outcome included duration of MV. MODS was assessed using the Sequential Organ Failure Assessment score [17]. Health-related quality of life was measured by the Veterans RAND 12 Item Health Survey [18, 19]. Functional status was measured by the Activities of Daily Living Scale and the Instrumental Activities of Daily Living Scale [20, 21]. Outcome definitions are detailed in the protocol.
Statistical analysis
We estimated that 1406 participants randomized 1:1 to the two treatment groups would provide 85% power to detect a 7% absolute reduction in 60-day mortality (21% in the methylprednisolone group vs. 28% in the placebo group). The original plan was to randomize 1420 participants (accounting for 1% attrition in primary outcome) over 5 years (January 2012–December 2016) and conduct two interim analyses at approximately 50% and 75% of the target number of participants to allow early discontinuation for efficacy (based on two-sided boundaries[22]) or futility (based on conditional power). Because of low recruitment, an ad hoc interim futility analysis was conducted on April 8, 2015 based on data as of February 6, 2015. At that time, 431 participants were randomized and the primary outcome was available for 372 participants. A one-sided non-binding futility boundary was calculated [22]. Conditional power was calculated for a range of differences in 60-day mortality (0–10%) and for two different target numbers of patients with 60-day mortality (the original target 1406 and the projected sample size 800 by December 2016). Based on the information, the DMC supported continued recruitment until the end of the planned recruitment period (December 2016). Study enrollment was stopped on April 30, 2016 due to persistent low recruitment; the final number of randomizations was 586. No additional interim analysis was done. Study follow-up ended in August 2016, which allowed collection of primary outcomes for all randomized participants. We report data for 584 participants, because two participants were improperly consented, and their data cannot be used for analyses.
Primary analyses were performed on the intention-to-treat sample (n = 584). Sixty-day all-cause mortality was compared by Chi-square test. The difference in percentages of 60-day mortality and the 95% confidence interval (CI) were calculated. Generalized linear mixed effect models were used to adjust for site (as a random effect) and baseline patient characteristics (as fixed effects), including MV status at randomization, age, Acute Physiology and Chronic Health Evaluation (APACHE) III score, bacteremia, use of anti-inflammatory medications, and use of macrolide antibiotics at baseline. Pneumonia Severity Index [23] class and Simplified Acute Physiology Score (SAPS) III score [24] were not included in the model to reduce collinearity of the covariates. Sensitivity analyses were performed to assess robustness of results and included using the per-protocol sample and different imputation methods for 21 participants missing primary outcomes (all due to early study withdrawal). The results from imputations were similar and not shown. Kaplan–Meier estimate of survival probability at day 60 was also calculated. Pre-specified subgroup analyses included MV status at randomization, APACHE III score quartiles, and CAP versus HCAP; post hoc subgroup analyses included severity of CAP, adequacy of initial antibiotic treatment, ARDS at baseline, and time of study treatment initiation (within 48 h vs. > 48 h of hospital presentation). Logistic regression was used to examine subgroups by treatment interactions.
Secondary outcomes were compared using Chi-square test or Fisher’s exact test for categorical outcomes, two-sample t tests or Wilcoxon rank-sum tests for continuous outcomes, and log rank tests and Kaplan–Meier curves for time to death and duration of MV up to day 28. Survival up to 180 days was compared by the restricted mean survival time (RMST) [25].
All p values are two-sided. The p values for secondary and exploratory outcomes were adjusted for multiplicity by the Bonferroni method, separately for in-hospital outcomes and post-discharge outcomes. The widths of the confidence intervals for the treatment differences in secondary and exploratory outcomes were not adjusted for multiplicity. SAS 9.4 (SAS Institute, Cary, NC, USA) was used for analysis. Unless specified otherwise, results are reported as methylprednisolone vs. placebo.
Results
Patients
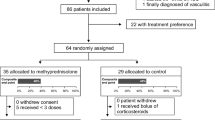
Of the 3936 patients who were assessed for eligibility, 584 were randomized; 70% were randomized within 48 h of hospital presentation and 94% within 72 h (median time to randomization, 37 h). Two hundred and ninety-seven participants were assigned to the methylprednisolone group and 287 to the placebo group (Fig. 1); 193 (33%) were receiving MV at the time of randomization. A total of 382 (65%) participants started study treatment within 48 h of hospital presentation and 513 (88%) within 72 h (median time from hospital presentation to study treatment initiation, 40 h). The study flow diagram is shown in Fig. 1, which also provides information on study drug withdrawal and reasons.
Enrollment, randomization, and follow-up. ¥Participants who were consented improperly are not included in this diagram. §The reasons for failing eligibility criteria were “select all that apply,” so one patient may have more than one reason for exclusion. Five patients who did not meet eligibility criteria (three did not meet inclusion criteria and two met exclusion criteria) were randomized. *Reasons for study drug withdrawal were check all that apply. ¶Active gastrointestinal bleeding requiring transfusion of at least 5 units of PRBC’s. ¶¶Such as exacerbation of COPD or asthma, and vasculitis
The two treatment groups were balanced in demographics and baseline patient characteristics (Table 1). The mean age was 68.8 years, 96% were male, and 83% were White. Patients had an average of four major comorbidities (Table S1). Thirty-four percent of participants met HCAP criteria, 69% had multi-lobar involvement on chest radiograph, 15% had bacteremia, 11% had ARDS at enrollment, and 13% had vasopressor-dependent shock at enrollment. Pathogens potentially responsible for the pneumonia were identified in 250 (43%) of the 577 participants with specimens from the respiratory tract, pleural fluid, blood or urine. The most common pathogens isolated were Staphylococcus aureus (10%), Streptococcus pneumoniae (9%), Pseudomonas aeruginosa (3%), and Escherichia coli (3%). Initial antibiotic treatment was deemed adequate in 96% of the participants based on ATS/IDSA guideline recommendations (Fig. 2).
Kaplan–Meier estimate of survival. Kaplan–Meier estimates of survival are shown in the overall population (A), in patients who were receiving mechanical ventilation at randomization (Patients on MV; B), and in those not receiving mechanical ventilation at randomization (Patients not on MV; C). The inset in each panel shows the same data on an enlarged y axis and up to day 60
Primary outcome
There was no significant difference in 60-day all-cause mortality (16% vs. 18%; unadjusted absolute risk difference − 2%, 95% CI − 8 to 5%; unadjusted odds ratio (OR) 0.89, 95% CI 0.58–1.38; p = 0.61) (Table 2). The result was similar when adjusted for site and MV status at randomization (adjusted OR 0.90; 95% CI 0.57–1.40; p = 0.63) and when also adjusted for baseline patient characteristics (adjusted OR, 0.87; 95% CI 0.53–1.42; p = 0.58). Kaplan–Meier estimate of 60-day mortality was 16% (95% CI 12–21%) in the methylprednisolone group and 18% (95% CI 14–23%) in the placebo group. No significant variation was found in the treatment effect across study sites. Results were similar in the per-protocol sample (Table S2 and Table S3). There was no significant between-group difference in the subgroup analyses (Table S4 and Fig. 3).
60-day all-cause mortality according to subgroup. The odds ratios and 95% confidence intervals are based on logistic regression with treatment as the single covariate. The widths of the confidence intervals have not been adjusted for multiplicity and therefore cannot be used to infer treatment effects
Secondary outcomes
In-hospital morbidity and mortality
There were no significant differences between the treatment groups in development of vasopressor-dependent shock, development of ARDS, MV-free days up to days 8 or 28, duration of ICU stay (median 3 vs. 4 days; p = 1.00), duration of hospital stay (median 7 vs. 8 days; p = 1.00), or hospital mortality (12% vs. 10%; p = 1.00) (Table 2). Among the 25 (12 vs. 13) participants who developed new shock or ARDS, 5 (1 vs. 4) stopped study medication to receive open label glucocorticoid treatment. Among participants who required MV at randomization, there was a 3-day reduction in median duration of MV (median 4 vs. 7 days; hazard ratio (HR) 1.44; 95% CI 1.04–1.99; p = 0.21 after Bonferroni correction).
Post-discharge morbidity and mortality
There were no significant between-group differences in cardiovascular complications, quality of life, functional status, or re-hospitalizations (Table 2). The most common reasons for re-hospitalization were pneumonia (20%), congestive heart failure (18%), and chronic obstructive pulmonary disease (COPD) (17%).
The two treatment groups had similar 180-day mortality (21% vs. 24%; OR 0.86; 95% CI 0.58–1.29; p = 1.00) and RMST up to day 180 (151 days vs. 149 days; difference 2.5 days; 95% CI − 7.7 to 12.6 days; p = 1.00) (Table 2). Kaplan–Meier estimate of mortality by 180 days was 20% (95%CI, 16% to 26%) in the methylprednisolone group and 23% (95% CI 19–29%) in the placebo group. The two groups also had similar 1-year mortality (30% vs. 33%; OR 0.88; 95% CI 0.61–1.27; p = 1.00) and time to death (HR 0.90; 95% CI 0.66–1.22; p = 1.00) (Table 2 and Fig. 2A). Results of secondary outcomes were similar in the per-protocol sample (Table S2) and within the MV (Table S5 and Fig. 2B) and non-MV strata (Table S6 and Fig. 2C). Within each stratum, the two treatment groups had similar baseline characteristics (Table S7 and Table S8).
Cause of death
No apparent between-group differences in immediate or underlying cause of death were observed for all deaths, deaths up to day 60, deaths during initial hospitalization, or deaths after discharge from initial hospitalization (Tables S9 and S10).
Adverse events
During the 180 days after randomization, 365 SAEs occurred in 167 (56.2%) participants in the methylprednisolone group, and 342 SAEs occurred in 162 (56.4%) participants in the placebo group (Table S11). There were no significant differences between treatment arms in SAEs (Table S11) or complications (Table S12) during 180 days after randomization or in in-hospital or post-discharge complications (data not shown).
Discussion
The ESCAPe trial showed that, in participants admitted to the ICU with severe CAP or HCAP, a 20-day treatment with low-dose methylprednisolone did not significantly reduce all-cause 60-day mortality, the primary outcome. We observed a 3-day reduction in median duration of MV in participants who required MV at randomization, although the certainty of this finding may be low given the small sample size in this subgroup, the imprecision of the estimated difference, and lack of multiplicity correction. No other significant differences were found in morbidity or mortality outcomes or complications during 1 year of follow-up.
To our knowledge, this is the largest trial investigating the efficacy of adjunct glucocorticoids on patients with severe pneumonia requiring ICU admission and the first randomized controlled trial (RCT) designed to evaluate both short- and long-term outcomes. We review our findings in the context of recent literature. In the last 15 years, 11 published RCTs investigated prolonged glucocorticoid treatment in patients hospitalized with bacterial CAP (n = 1808) [11, 26]; six of the largest RCTs (n = 1506) were part of an individual patient data meta-analysis [27].
We did not find a significant reduction in 60-day mortality or mortality up to 1 year, which is contrary to the observed reduction in 30-day mortality in severe CAP meta-analyses [11, 26]. The timing for glucocorticoid administration in this study may have missed the optimal window for intervention. Our study allowed for randomization up to 72–96 h after hospital admission. While 65% of study participants initiated study treatment within 48 h of hospital presentation and 88% within 72 h, the inherent delay in the initiation of anti-inflammatory therapy occurred during the initial peaks of inflammatory mediators in response to invasive microbial pathogens [28] and may have attenuated potential benefits [29]. Second, the methylprednisolone dose of 40 mg/day may be inadequate to achieve the level of glucocorticoid receptor saturation necessary for optimal anti-inflammatory response; a higher dose was found effective in ARDS (most attributed to pneumonia) [30]. Third, compared to the prior largest RCT on severe CAP [31], our patient population was sicker, as evidenced by oxygenation indices, need for MV, and a greater burden of comorbidities associated with glucocorticoid resistance such as chronic pulmonary and cardiovascular diseases [32]. Fourth, the observed mortality in the control group was substantially lower than what was used for the power calculation. Fifth, the broad range of severity across our study cohort likely represented different pathophysiologic processes of which corticosteroids possibly have a heterogeneous effect.
For secondary and exploratory outcomes, the 1-day reduction in median hospitalization duration (95% CI − 2.3 to 0.3 days) was similar to that reported in meta-analysis [27] and mainly driven by a 2.6-day reduction in the MV stratum (95% CI − 6.2 to 1.1 days). Contrary to prior investigations, we did not observe significant reduction in progression to shock or ARDS [26], increased risk for re-hospitalization [27], or lower myocardial infarction incidence [33].
The longer duration of methylprednisolone treatment in our trial was not associated with an increased risk of SAEs or complications within 180 days after randomization. These findings are consistent with those of updated meta-analyses of ICU patients with pneumonia [26], septic shock [34], and ARDS [30], underscoring the safety of prolonged glucocorticoid treatment in this population.
Response to glucocorticoid treatment may be affected by the severity of dysregulated systemic inflammation [31, 35, 36]. In a RCT in patients with severe CAP and C-reactive protein (CRP) levels > 150 mg/L, methylprednisolone was found to reduce treatment failure [31]. In a retrospective cohort study in patients with severe CAP admitted to ICU and receiving glucocorticoid treatment, the subgroup with CRP levels > 150 mg/L had faster recovery of hypoxemia and increased ICU- and hospital-free days [35]. These findings suggest that biologic markers may help identify patients most likely to benefit from glucocorticoid treatment. The blood samples collected in ESCAPe will allow examination of the relationship between clinical outcomes and markers of systemic inflammation over time, which may provide the groundwork for development of personalized glucocorticoid treatment strategies [30].
Evidence of glucocorticoid benefits in severe pneumonia due to coronavirus disease 2019 (COVID-19) [37, 38] and ARDS [39] has generated greater interest in this field of research. The safety of prolonged methylprednisolone treatment has been confirmed [11, 26, 27]. However, notable treatment heterogeneity in published protocols [30], such as the specific glucocorticoid, timing of initiation, dosage, duration, mode of administration, and tapering strategy, underscore the need for a more uniform approach. Further studies are required to clarify how these treatment components impact clinical outcomes and host responses. During the pandemic, variability in response to glucocorticoid treatment was observed, leading clinicians to adjust dosage and duration based on markers of inflammation and oxygenation. This has called attention to an underappreciated aspect of glucocorticoid treatment, the great interindividual variability in (i) achieved blood drug levels [40] and (ii) intracellular glucocorticoids receptor sensitivity [41], areas in need of research [30].
This trial has several limitations. First, enrollment was stopped before reaching the target sample size 1420 because of low recruitment. The main contributing factor to low recruitment was that the proportion of the patient population meeting study eligibility criteria was lower than anticipated (26% versus anticipated 70%), even though the consent rate for eligible patients was higher than anticipated (57% versus anticipated 30%). Another contributing factor was 2 years of relatively low influenza activity during the recruitment period. Second, the certainty of our overall study findings may be limited given that the sample size was lower than target and the analyses may be underpowered. Third, delayed initiation of anti-inflammatory therapy may have attenuated the differences between the treatment groups [29]. Fourth, the VA population is predominantly older, male, and with multiple comorbidities compared to the general population [27]; therefore, the trial’s results may not be generalizable to non-Veterans. Fifth, the high proportion of patients excluded due to physician opinion of not being a viable candidate might indicate a potential risk of selection bias. Sixth, this study excluded patients with recent or concurrent use of glucocorticoids; thus, it cannot determine if patients with severe CAP who require a short course of glucocorticoids for co-morbid diseases (such as COPD) would benefit from prolonged glucocorticoid treatment.
Conclusion
In patients with severe CAP, prolonged low-dose methylprednisolone treatment did not significantly reduce 60-day mortality. The risk for complications was similar to the control group.
Data sharing statement
See Supplement Appendix.
References
Mayr FB, Yende S, Angus DC (2014) Epidemiology of severe sepsis. Virulence 5:4–11
Kang C-I, Song J-H, Kim SH, Chung DR, Peck KR, Thamlikitkul V, Wang H, So TM-K, Hsueh P-R, Yasin RM (2013) Risk factors and pathogenic significance of bacteremic pneumonia in adult patients with community-acquired pneumococcal pneumonia. J Infect 66:34–40
Cillóniz C, Polverino E, Ewig S, Aliberti S, Gabarrús A, Menéndez R, Mensa J, Blasi F, Torres A (2013) Impact of age and comorbidity on cause and outcome in community-acquired pneumonia. Chest 144:999–1007
Hsu JL, Siroka AM, Smith MW, Holodniy M, Meduri GU (2011) One-year outcomes of community-acquired and healthcare-associated pneumonia in the Veterans Affairs Healthcare System. Int J Infect Dis 15:e382-387
Musher DM, Montoya R, Wanahita A (2004) Diagnostic value of microscopic examination of Gram-stained sputum and sputum cultures in patients with bacteremic pneumococcal pneumonia. Clin Infect Dis 39:165–169
Corrales-Medina VF, Musher DM (2011) Immunomodulatory agents in the treatment of community-acquired pneumonia: a systematic review. J Infect 63:187–199
Iwashyna TJ, Ely EW, Smith DM, Langa KM (2010) Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA, J Am Med Assoc 304:1787–1794
Wang HE, Szychowski JM, Griffin R, Safford MM, Shapiro NI, Howard G (2014) Long-term mortality after community-acquired sepsis: a longitudinal population-based cohort study. BMJ Open 4:e004283
Confalonieri M, Meduri GU (2011) Glucocorticoid treatment in community-acquired pneumonia. Lancet 377:1982–1984
Yende S, Kellum JA, Talisa VB, Palmer OMP, Chang C-CH, Filbin MR, Shapiro NI, Hou PC, Venkat A, LoVecchio F (2019) Long-term host immune response trajectories among hospitalized patients with sepsis. JAMA Netw Open 2:e198686–e198686
Siemieniuk RA, Meade MO, Alonso-Coello P, Briel M, Evaniew N, Prasad M, Alexander PE, Fei Y, Vandvik PO, Loeb M, Guyatt GH (2015) Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med 163:519–528
Briel M, Spoorenberg SM, Snijders D, Torres A, Fernandez-Serrano S, Meduri GU, Gabarrús A, Blum CA, Confalonieri M, Kasenda B (2017) Corticosteroids in patients hospitalized with community-acquired pneumonia: systematic review and individual patient data metaanalysis. Clin Infect Dis 66:346–354
Meduri GU, Chrousos GP (2020) General adaptation in critical illness: glucocorticoid receptor-alpha master regulator of homeostatic corrections. Front Endocrinol (Lausanne) 11
Nawab Q, Golden E, Confalonieri M, Umberger R, Meduri G (2011) Corticosteroid treatment in severe community-acquired pneumonia: duration of treatment affects control of systemic inflammation and clinical improvement. Intensive Care Med 37:1153–1554
Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM Jr, Musher DM, Niederman MS, Torres A, Whitney CG (2007) Infectious diseases society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 44(Suppl 2):S27-72
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL (2008) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med 34:17–60
Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL (2001) Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 286:1754–1758
Kazis LE, Selim AJ, Rogers W, Ren XS, Lee A, Miller D, Veterans RAND 12 item Health Survey (VR-12): a white paper summary
Selim AJ, Rogers W, Fleishman JA, Qian SX, Fincke BG, Rothendler JA, Kazis LE (2009) Updated US population standard for the Veterans RAND 12-item Health Survey (VR-12). Qual Life Res 18:43–52
Lawton MP, Brody EM (1969) Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9:179–186
Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW (1963) Studies of illness in the aged. The Index of Adl: a standardized measure of biological and psychosocial function. JAMA 185:914–919
Lai TL, Shih M-C (2004) Power, sample size and adaptation considerations in the design of group sequential clinical trials. Biometrika 91:507–528
Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, Coley CM, Marrie TJ, Kapoor WN (1997) A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 336:243–250
Moreno RP, Metnitz PG, Almeida E, Jordan B, Bauer P, Campos RA, Iapichino G, Edbrooke D, Capuzzo M, Le Gall JR (2005) SAPS 3–from evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med 31:1345–1355
Zhao L, Tian L, Uno H, Solomon SD, Pfeffer MA, Schindler JS, Wei LJ (2012) Utilizing the integrated difference of two survival functions to quantify the treatment contrast for designing, monitoring, and analyzing a comparative clinical study. Clin Trials 9:570–577
Pastores SM, Annane D, Rochwerg B (2018) Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (part II): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Intensive Care Med 44:474–477
Briel M, Spoorenberg SMC, Snijders D, Torres A, Fernandez-Serrano S, Meduri GU, Gabarrus A, Blum CA, Confalonieri M, Kasenda B, Siemieniuk RAC, Boersma W, Bos WJW, Christ-Crain M, Ovidius Study G, Capisce Study G, Group SS (2018) Corticosteroids in patients hospitalized with community-acquired pneumonia: systematic review and individual patient data metaanalysis. Clin Infect Dis 66:346–354
Coelho LM, Salluh JI, Soares M, Bozza FA, Verdeal JC, Castro-Faria-Neto HC, Lapa-e-Silva JR, Bozza PT, Povoa P (2012) Patterns of c-reactive protein RATIO response in severe community-acquired pneumonia: a cohort study. Crit Care 16:R53
Monedero P, Gea A, Castro P, Candela-Toha AM, Hernandez-Sanz ML, Arruti E, Villar J, Ferrando C, Network C-SI (2021) Early corticosteroids are associated with lower mortality in critically ill patients with COVID-19: a cohort study. Crit Care 25:2
Meduri GU, Annane D, Confalonieri M, Chrousos GP, Rochwerg B, Busby A, Ruaro B, Meibohm B (2020) Pharmacological principles guiding prolonged glucocorticoid treatment in ARDS. Intensive Care Med 46:2284–2296
Torres A, Sibila O, Ferrer M, Polverino E, Menendez R, Mensa J, Gabarrus A, Sellares J, Restrepo MI, Anzueto A, Niederman MS, Agusti C (2015) Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 313:677–686
Rodriguez JM, Monsalves-Alvarez M, Henriquez S, Llanos MN, Troncoso R (2016) Glucocorticoid resistance in chronic diseases. Steroids 115:182–192
Cangemi R, Falcone M, Taliani G, Calvieri C, Tiseo G, Romiti GF, Bertazzoni G, Farcomeni A, Violi F, Group SS (2019) Corticosteroid use and incident myocardial infarction in adults hospitalized for community-acquired pneumonia. Ann Am Thorac Soc 16:91–98
Annane D, Pastores S, Rochwerg B, Arlt W, Balk R, Beishuizen A, Briegel J, Carcillo J, Christ-Crain M, Cooper M (2017) Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Crit Care Med 45:2078–2088
Odeyemi YE, Herasevich S, Chalmers SJ, Barreto EF, Frank RD, Gajic OO, Yadav H (2020) Biomarker-concordant steroid use in critically ill patients with pneumonia. Mayo Clin Proc Innov Qual Outcomes 4:649–656
Li J, Liao X, Zhou Y, Wang L, Yang H, Zhang W, Zhang Z, Kang Y (2021) Comparison of associations between glucocorticoids treatment and mortality in COVID-19 patients and SARS patients: a systematic review and meta-analysis. Shock 56:215–228
Recovery Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ (2021) Dexamethasone in hospitalized patients with COVID-19. N Engl J Med 384:693–704
World Health Organization (2020) Corticosteroids for COVID-19: living guidance, 2 September 2020. In: Book corticosteroids for COVID-19: living guidance, 2 September 2020. World Health Organization, City
Villar J, Ferrando C, Martinez D, Ambros A, Munoz T, Soler JA, Aguilar G, Alba F, Gonzalez-Higueras E, Conesa LA, Martin-Rodriguez C, Diaz-Dominguez FJ, Serna-Grande P, Rivas R, Ferreres J, Belda J, Capilla L, Tallet A, Anon JM, Fernandez RL, Gonzalez-Martin JM, Dexamethasone in AN (2020) Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med 8:267–276
Yates CR, Vysokanov A, Mukherjee A, Ludden TM, Tolley EA, Meduri GU, Dalton JT (2001) Time-variant increase in methylprednisolone clearance in patients with acute respiratory distress syndrome: a population pharmocokinetic study. J Clin Pharmacol 41:1–10
Chriguer RS, Elias LLK, da Silva Jr IM, Vieira JGH, Moreira AC, de Castro M (2005) Glucocorticoid sensitivity in young healthy individuals: in vitro and in vivo studies. J Clin Endocrinol Metab 90:5978–5984
Acknowledgements
We dedicate this work to the memory of Timothy D. Bigby, Principal Investigator at the San Diego VA; his warmth, kindness and professional commitment is missed by all those that had the privilege of working with him. Additional recognition goes to Kelvin K. Lee, PhD, Valerie McGuire, PhD, and Colleen Fitzsimmons (VA Palo Alto Cooperative Studies Program Coordinating Center), Stuart Warren, David Pittman and Jolene Day (VA Albuquerque Cooperative Studies Program Pharmacy Coordinating Center), and Jennifer Ransom, MPH, CCRP (Memphis VA Medical Center Chair’s Office). We also wish to thank the Veterans whose participation made the study possible.
ESCAPe study group members: Khalid Bashir, Octavian Ioachimescu, Theresa Buck, David Johnson, Ali El Solh, Michael Frye, Ralph Panos, Mohammad Shatat, Enoch Gray, Brian Smith, Myron Kung, James Cutrell, Roger Bedimo, Peruvemba Sriram, Charlie Lan, Padmashi Rastogi, John Callaghan, Chadi Hage, Mark Plautz, Takako Schaninger, Richard Greenberg, Lennard Specht, Catherine Sassoon, Juan Guardiola, Julio Ramirez, Muthiah P. Muthiah, Roland Schein, Andreea Antonesu-Turcu, Kathryn Rice, Houssein Youness, Lee Morrow, Ware Kuschner, Lilibeth Pineda, Richard Allen Robbins, Sharon Camhi, Matthew Jankowich, Waseem Ahmed, Thomas Martin, Mitchell Horowitz, John Nord, Mark Elstad, Marcos I. Restrepo, Antonio Anzueto, Timothy Bigby, William Rodriguez-Cintron, Vincent Fan, Pratibha Kaul, Michael Habib, Nitin Seam, Guy Soo Hoo.
Veterans affairs medical centers participating in the ESCAPe trial: Participating VAMCs in alphabetical order (ESCAPe site personnel in parenthesis): Asheville, (Khalid Bashir, Whitney Sprinkle, Webster Bazemore, Sujatha Goli, John Lucke, Valerie Allen); Atlanta, (Octavian Ioachimescu, Tiffany Elliott, Amy Anderson, Joanne Allam, Ashish Mehta, Patricia Noren, Vidisha Tanukonda); Bay Pines, (Theresa Buck, David Johnson, Caitlin Butler, James Blankenship, Patricia Ellis, Minhaj Siddiqui, Lynn Anderson); Buffalo, (Ali El-Solh, Lynne Frydrych, Karin Provost, Rachel LaPorta, Philippe Jaoude, Leah Vermont); Charleston, (Michael Frye, Leslie Harrell); Cincinnati, (Ralph Panos, Laura Lach, Dennis McGraw, Nishant Gupta, William Eschenbacher); Cleveland, (Mohammad Shatat, Kimberley Byrne, Frank Jacono, Puja Van Epps); Columbia, (Enoch Gray, Brian Smith, Myron Kung, Joanna Snead, Justin Reynolds, Mohammed Wallam, Shilpa Patel, Stuart Smith, Kathryn Mason, Heather Roth, Rebecca Warner); Dallas, (James Cutrell, Roger Bedimo, John Battaile, Teagan Lampkin, Joyce Ghormley, David Albracht, Cyenthia Willis, Padmashri Rastogi, Holly Wise); Gainesville, (Peruvemba Sriram, Omerea Herring, Daniel Urbine, James Wynne, Ramanjeet Singh, Alice Boyette, Rosie Kizza, Eloise Harman, Michelle Ginsburg); Houston, (Charlie Lan, Sarah Perusich, Daniel Musher, Farrah Kheradmand, Lavannya Pandit, Roberto Casal, Rolando Rumbaut, Amir Sharafkhaneh, Suryakanta Velamuri, Pamela Smithwick, Sherilyn Pillack, Nazanin Zarinkamar, Emily Broussard, Cynthia Boudreaux); Indianapolis, (John Callaghan, Sharon Henson, Casey Stahlheber, Vru Patel, Aliya Noor); Kansas City, (Mark Plautz, Cheryl Perkins, Trenton Nauser, Justine Unruh); Lexington, (Takako Schaninger, Edward Hirshowitz, Hannah Ferrell, Sabrina Broden); Loma Linda, (Lennard Specht, Laura Weaver-Carnahan, James Anholm, Hemad Parekh, Nagamani Dandamudi, Kathleen Ellstrom, Jamie Portillo, Sara Rubio); Long Beach, (Catherine Sassoon, Romena Helal, Yih-Ming Chi, Farhad Mazdisnian, Aliya Asghar, David Stansbury, Gelincik Oraciklar, Lei Zhu); Louisville, (Juan Guardiola, Belica Graf, Rafael Perez, Bogdan Moldoveanu, Julio Ramirez, Jesse Roman, Umair Guahar, Angela Kain, Jinesh Mehta, Ginny Sciortino); Memphis, (Muthiah P. Muthiah, Kathleen Bockhold, Ibrahim Sultan-Ali, Amado Freire, Muhammad Zaman, Veronica Castillo, Margaret Sanders, Lori Sykes); Miami, (Roland Schein, Jacqueline Junco, Lisa Camacho, Cynthia Cely, Michael Hyre, Gregory McGrath, Daniel Coes, Devora Khan); Milwaukee, (Andreea Antonescu-Turcu, Susan Framberg, Mary Ellis, Sharon Pecsi, Christa Kallio); Minneapolis, (Kathryn Rice, Linda Kollman, Dennis Niewoehner, Angela Fabbrini, Debra Condon, Christine Wendt, Erin Wetherbee, Miranda Deconcini, Brandon Doyle, George Sarosi, Ken Kunisaki, Doris Stuber), Oklahoma, (Houssein Youness, Laurel Howard, Jean Keddissi, Ahmed Awab, Ashley Ellis); Omaha, (Lee Morrow, Mary Pote, Kristina Bailey, Daniel Hershberger, Theresa Ramelb); Palo Alto, (Ware Kuschner, Elena Nikolaev, Juliana Barr, Edward Bertaccini, Karen Bratcher, Carlos Brun, Rajinder Chitkara, Jon-Erik Holty, Geoffrey Lighthall, Daphne Ly, Harman Paintal, Craig Zone, Alda Vicencio); Phoenix, (Lilibeth Pineda, Susan Brannan, Clement Singarajah, Pat Jacobs, Lisa Orozco); Pittsburgh, (Sharon Camhi, Marie Berger, Kathryn Hartwig, Charles Atwood, Sarita Kumari, Emily Grum, Mary Van Buskirk, Marcia Homer, Melissa Clark); Providence, (Matthew Jankowich, Sandra Befera, Linda Nici, Sharon Rounds); Reno, (Waseem Ahmed, Anne Inda, Anurag Mehta, Pam Lehman, Andrew Liu, Claudine Aguilera); Salem, (Thomas Martin, Mary Halling, Anil Agarwal, David Boone, Barbara Bowers, Neeraj Gupta, Mitchell Horowitz, Deepa Lala, Mitra Sahebazamani, Tonda Yates); Salt Lake City, (John Nord, Amy Beckstead, Elisabeth Carr, Moises Cortez, Heather Dulin, Mark Elstad, Lydia Rosenthal, Karl Sanders, Lindsay Carpenter, Wade Brown); San Antonio, (Marcos I. Restrepo, Luis Reyes, Antonio Anzueto, Alejandro Arango, Sandra Adams, Anisha Arora, Karla Diaz, Felipe Fernandez, Pamela Foltz, Tim Hernandez, Timothy Houlihan, Stephanie Levine, Adriel Malave, Breion Mallioux, Diego Maselli, Benjamin Michels, Anoop Nambiar, Hector Payan, Jay Peters); San Diego, (Timothy Bigby*, Keith Nicalo, Judd Landsberg, Mark Fuster, Philippe Montgrain, Danielle Beck), San Juan, (William Rodriguez-Cintron, Julia Roig, Damaris Acosta-Miranda, Edwin Alicea, Joan Albors-Sanchez, Jesse Aleman-Ortiz, Felicita Aruz, Onix Cantres-Fonseca, Maria del Mar Torres-Perez, Francisco Del Olmo, Juan Flores, Sandra Galarza-Vargas, Ricardo Hernandez-Castillo, Juancarlos Martinez, Carlos Robles-Arias, Yarilys Rodriguez-Sepulveda); Seattle, (Vincent Fan, Cynthia Lotane, Pauline Shull, Richard Goodman, Cornelia Schneider); Syracuse, (Pratibha Kaul, Autum Bellinger, Girish Trikha, Adnan Abbasi, Bonnie VerHeecke, Mark Chilton); Tucson, (Michael Habib, Tom Vincent, Gina Blackwell, Dena L’Heureux, Wei Shen); Washington DC, (Nitin Seam, Parisa Coffman, Bashar Albarghuthy, Jessica Smith); West Los Angeles, (Guy Soo Hoo, May Luciano).
Member of committees and CSP 574 organizational structure of the trial: Planning Committee: Gianfranco Umberto Meduri, MD (Principal Proponent: Memphis VA Medical Center); Antonio Anzueto, MD (University of Texas Health Science Center); Mark Holodniy, MD (VA Palo Alto Health Care System); Philip Lavori, PhD (Stanford Comprehensive Cancer Center); Linda Nichols, PhD (Memphis VA Medical Center); Lori Nielsen (VA Palo Alto Cooperative Studies Program Coordinating Center); Mei-Chiung Shih, PhD (VA Palo Alto Cooperative Studies Program Coordinating Center); Anthony F. Suffredini, MD (National Institutes of Health Clinical Center); Derek C. Angus, MD, MPH (University of Pittsburgh School of Medicine); Thomas M. File, Jr., MD, MSc (Northeastern Ohio Universities College of Medicine); Kelvin K. Lee, PhD (VA Palo Alto Cooperative Studies Program Coordinating Center); Paul Marik, MD (East Virginia Medical School); Dennis E. Niewoehner, MD (Minneapolis VA Healthcare System); Mike Sather, PhD (VA CSP Clinical Research Pharmacy Coordinating Center, Albuquerque, NM); Mark W. Smith, PhD (VA Health Economics Resource Center); Stuart R. Warren, JD, PharmD (VA CSP Clinical Research Pharmacy Coordinating Center, Albuquerque, NM).
Study chairman’s office: Gianfranco Umberto Meduri, MD (Memphis VA Medical Center); Lisa Bridges, RN, MS, ACNP-BC (Memphis VA Medical Center); Jennifer Ransom, MPH (Memphis VA Medical Center).
Executive committee: Gianfranco Umberto Meduri, MD (Memphis VA Medical Center); Lisa Bridges, RN, MS, ACNP-BC (Memphis VA Medical Center); Ali El-Solh, MD (VA Western New York Health Care System); Nitin Seam, MD (National Institutes of Health Clinical Center); Antonio Anzueto, MD (University of Texas Health Science Center); Anthony F. Suffredini, MD (National Institutes of Health Clinical Center); Reba Umberger, MS, RN, CCRN, PhD (University of Tennessee Health Science Center); W. Andrew Bell, PharmD (Memphis VA Medical Center, Renown Health, Reno, NV); Anne Davis-Karim, PharmD and Stuart R. Warren, JD, (ex officio) (VA CSP Clinical Research Pharmacy Coordinating Center); Thomas J. Martin, MD (Salem VA Medical Center); Mei-Chiung Shih, PhD, Colleen Fitzsimmons, BS, Kelvin Lee, PhD, (ex officio), Julia Lin, PhD, (ex officio), Valerie McGuire, PhD, (ex officio), Lori Nielsen, BA, (ex officio) (VA CSP Palo Alto Coordinating Center).
Data and Safety Monitoring Committee: Robert Danner, MD (National Institutes of Health Clinical Center); Matthew Goetz, MD (VA Greater Los Angeles Healthcare System); Joan Hilton, ScD (University of California San Francisco); John Kellum, MD (University of Pittsburgh School of Medicine); David Looney, MD, PhD (University of California San Diego); Colin Wu, PhD (National Heart Lung and Blood Institute); Richard ZuWallack, MD (Saint Francis Hospital and Medical Center).
Clinical Evaluation Committee: Ali El-Solh, MD (VA Western New York Health Care System); Nitin Seam, MD (National Institutes of Health Clinical Center); Marcos I. Restrepo, MD (South Texas Veterans Health Care System); Antonio Anzueto, MD (University of Texas Health Science Center); Octavian Ioachimescu, MD (Atlanta VA Health Care System); Charlie Lan, MD (Michael E. DeBakey VA Medical Center).
VA Palo Alto CSP Coordinating Center: Mei-Chiung Shih, PhD, Ying Lu, PhD, Kelvin Lee, PhD, Valerie McGuire, PhD, Julia Lin, PhD, Lan Zhao, Colleen Fitzsimmons, Lori Nielsen, Parinaz Lajevardi, MPH, Aileen Baylosis, Elizabeth Smith, Lauren Uyeda, Ania Ray, Jennifer Cockroft, Martha Forrester, Aaron Alsleben, Anita Kelly, Nigel Gladhart.
VA Albuquerque CSP Clinical Research Pharmacy Coordinating Center: Stuart Warren, JD, PharmD, Anne Davis-Karim, PharmD, Alexandra Scrymgeour, PharmD, Jolene Day, and David Pittman.
The CSP Site Monitoring, Auditing and Review Team (SMART); Mary Mills, RN, CCRA, Tasha Anderson, RN, BSN, Mariette Coyle, RN, MSN, Barbara Curtis, Michelle Prehoda, Clair Haakenson, Darlene Kreuger.
Funding
This study was conducted with support from the VA Cooperative Studies Program. However, the views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or any US governmental agency. Role of the sponsor: The VA Cooperative Studies program was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation of the manuscript. The data from the 42 sites were maintained by the VA Cooperative Studies Program Coordinating Center in Palo Alto, CA, where Mei-Chiung Shih, PhD, performed all statistical analyses with assistance from Lan Zhao, MS, and Lauren Uyeda, MA. Funding was provided by Department of Veterans Affairs, Cooperative Studies Program (Grant no. CSP #2009).
Author information
Authors and Affiliations
Consortia
Contributions
GUM and M-CS had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the analyses. Study concept and design: GUM, M-CS, LB, NS, AD-K, MIR, AS, WAB, JL, and GDH. Acquisition of the data: TJM, AE-S, AA, PS, CL, MIR, JJG, TB, and DPJ. Analysis and interpretation of data: GUM and M-CS. Drafting of the manuscript: GUM and M-CS. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: M-CS, LZ, and LU. Obtaining funding: GUM and M-CS. Administrative, technical, or material support: GUM, M-CS, LB, JL, and LN.
Corresponding author
Ethics declarations
Conflicts of interest
No funding or other conflicts to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or any US governmental agency.
The members of the ESCAPe study group are listed in the Acknowledgements section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Meduri, G.U., Shih, MC., Bridges, L. et al. Low-dose methylprednisolone treatment in critically ill patients with severe community-acquired pneumonia. Intensive Care Med 48, 1009–1023 (2022). https://doi.org/10.1007/s00134-022-06684-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-022-06684-3