Abstract
Purpose
Guidelines recommend administering antibiotics within 1 h of sepsis recognition but this recommendation remains untested by randomized trials. This trial was set up to investigate whether survival is improved by reducing the time before initiation of antimicrobial therapy by means of a multifaceted intervention in compliance with guideline recommendations.
Methods
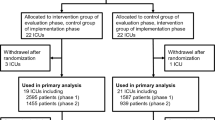
The MEDUSA study, a prospective multicenter cluster-randomized trial, was conducted from July 2011 to July 2013 in 40 German hospitals. Hospitals were randomly allocated to receive conventional continuous medical education (CME) measures (control group) or multifaceted interventions including local quality improvement teams, educational outreach, audit, feedback, and reminders. We included 4183 patients with severe sepsis or septic shock in an intention-to-treat analysis comparing the multifaceted intervention (n = 2596) with conventional CME (n = 1587). The primary outcome was 28-day mortality.
Results
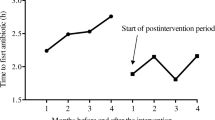
The 28-day mortality was 35.1% (883 of 2596 patients) in the intervention group and 26.7% (403 of 1587 patients; p = 0.01) in the control group. The intervention was not a risk factor for mortality, since this difference was present from the beginning of the study and remained unaffected by the intervention. Median time to antimicrobial therapy was 1.5 h (interquartile range 0.1–4.9 h) in the intervention group and 2.0 h (0.4–5.9 h; p = 0.41) in the control group. The risk of death increased by 2% per hour delay of antimicrobial therapy and 1% per hour delay of source control, independent of group assignment.
Conclusions
Delay in antimicrobial therapy and source control was associated with increased mortality but the multifaceted approach was unable to change time to antimicrobial therapy in this setting and did not affect survival.


Similar content being viewed by others
References
Levy MM, Artigas A, Phillips GS et al (2012) Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis 12:919–924. doi:10.1016/S1473-3099(12)70239-6
Kumar A, Roberts D, Wood KE et al (2006) Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34:1589–1596. doi:10.1097/01.CCM.0000217961.75225.E9
Larché J, Azoulay E, Fieux F et al (2003) Improved survival of critically ill cancer patients with septic shock. Intensive Care Med 29:1688–1695. doi:10.1007/s00134-003-1957-y
Gaieski DF, Mikkelsen ME, Band RA et al (2010) Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med 38:1045–1053. doi:10.1097/CCM.0b013e3181cc4824
Clec’h C, Timsit JF, De Lassence A et al (2004) Efficacy of adequate early antibiotic therapy in ventilator-associated pneumonia: influence of disease severity. Intensive Care Med 30:1327–1333. doi:10.1007/s00134-004-2292-7
Noritomi DT, Ranzani OT, Monteiro MB et al (2014) Implementation of a multifaceted sepsis education program in an emerging country setting: clinical outcomes and cost-effectiveness in a long-term follow-up study. Intensive Care Med 40:182–191. doi:10.1007/s00134-013-3131-5
Boyer A, Vargas F, Coste F et al (2009) Influence of surgical treatment timing on mortality from necrotizing soft tissue infections requiring intensive care management. Intensive Care Med 35:847–853. doi:10.1007/s00134-008-1373-4
Buck DL, Vester-Andersen M, Møller MH, Danish Clinical Register of Emergency Surgery (2013) Surgical delay is a critical determinant of survival in perforated peptic ulcer. Br J Surg 100:1045–1049. doi:10.1002/bjs.9175
Azuhata T, Kinoshita K, Kawano D et al (2014) Time from admission to initiation of surgery for source control is a critical determinant of survival in patients with gastrointestinal perforation with associated septic shock. Crit Care 18:R87. doi:10.1186/cc13854
Rhodes A, Evans LE, Alhazzani W et al (2017) Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. doi:10.1007/s00134-017-4683-6
Grimshaw J, Campbell M, Eccles M, Steen N (2000) Experimental and quasi-experimental designs for evaluating guideline implementation strategies. Fam Pract 17(Suppl 1):S11–S16
Ioannidis JP, Haidich AB, Pappa M et al (2001) Comparison of evidence of treatment effects in randomized and nonrandomized studies. JAMA 286:821–830. doi:10.1001/jama.286.7.821
Lilly CM (2014) The ProCESS trial—a new era of sepsis management. N Engl J Med 370:1750–1751. doi:10.1056/NEJMe1402564
Sterling SA, Miller WR, Pryor J et al (2015) The impact of timing of antibiotics on outcomes in severe sepsis and septic shock: a systematic review and meta-analysis. Crit Care Med 43:1907–1915. doi:10.1097/CCM.0000000000001142
Rhee C, Gohil S, Klompas M (2014) Regulatory mandates for sepsis care—reasons for caution. N Engl J Med 370:1673–1676. doi:10.1056/NEJMp1400276
Ivers N, Jamtvedt G, Flottorp S et al (2012) Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev 6:CD000259. doi:10.1002/14651858.CD000259.pub3
Shiramizo SCPL, Marra AR, Durão MS et al (2011) Decreasing mortality in severe sepsis and septic shock patients by implementing a sepsis bundle in a hospital setting. PLoS One 6:e26790. doi:10.1371/journal.pone.0026790
Tipler PS, Pamplin J, Mysliwiec V et al (2013) Use of a protocolized approach to the management of sepsis can improve time to first dose of antibiotics. J Crit Care 28:148–151. doi:10.1016/j.jcrc.2012.08.021
Ferrer R, Artigas A, Levy MM et al (2008) Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA 299:2294–2303. doi:10.1001/jama.299.19.2294
Garrouste-Orgeas M, Soufir L, Tabah A et al (2012) A multifaceted program for improving quality of care in intensive care units: IATROREF study. Crit Care Med 40:468–476. doi:10.1097/CCM.0b013e318232d94d
Scales DC, Dainty K, Hales B et al (2011) A multifaceted intervention for quality improvement in a network of intensive care units: a cluster randomized trial. JAMA 305:363–372. doi:10.1001/jama.2010.2000
van der Veer SN, de Vos MLG, van der Voort PHJ et al (2013) Effect of a multifaceted performance feedback strategy on length of stay compared with benchmark reports alone: a cluster randomized trial in intensive care. Crit Care Med 41:1893–1904. doi:10.1097/CCM.0b013e31828a31ee
Marsteller JA, Sexton JB, Hsu Y-J et al (2012) A multicenter, phased, cluster-randomized controlled trial to reduce central line-associated bloodstream infections in intensive care units. Crit Care Med 40:2933–2939. doi:10.1097/CCM.0b013e31825fd4d8
Writing Group for the CHECKLIST-ICU Investigators and the Brazilian Research in Intensive Care Network (BRICNet), Cavalcanti AB, Bozza FA et al (2016) Effect of a quality improvement intervention with daily round checklists, goal setting, and clinician prompting on mortality of critically ill patients: a randomized clinical trial. JAMA 315:1480–1490. doi:10.1001/jama.2016.3463
Bloos F, Thomas-Rüddel D, Rüddel H et al (2014) Impact of compliance with infection management guidelines on outcome in patients with severe sepsis: a prospective observational multi-center study. Crit Care 18:R42. doi:10.1186/cc13755
Hemming K, Girling AJ, Sitch AJ et al (2011) Sample size calculations for cluster randomised controlled trials with a fixed number of clusters. BMC Med Res Methodol 11:102. doi:10.1186/1471-2288-11-102
Wears RL (2002) Advanced statistics: statistical methods for analyzing cluster and cluster-randomized data. Acad Emerg Med 9:330–341
Grund S, Lüdtke O, Robitzsch A (2016) Multiple imputation of multilevel missing data. SAGE Open. doi:10.1177/2158244016668220
Miller RR, Dong L, Nelson NC et al (2013) Multicenter implementation of a severe sepsis and septic shock treatment bundle. Am J Respir Crit Care Med 188:77–82. doi:10.1164/rccm.201212-2199OC
Matthaeus-Kraemer CT, Thomas-Rueddel DO, Schwarzkopf D et al (2015) Barriers and supportive conditions to improve quality of care for critically ill patients: a team approach to quality improvement. J Crit Care 30:685–691. doi:10.1016/j.jcrc.2015.03.022
Scheer CS, Fuchs C, Kuhn S-O et al (2017) Quality improvement initiative for severe sepsis and septic shock reduces 90-day mortality: a 7.5-year observational study. Crit Care Med 45:241–252. doi:10.1097/CCM.0000000000002069
Cross G, Bilgrami I, Eastwood G et al (2015) The epidemiology of sepsis during rapid response team reviews in a teaching hospital. Anaesth Intensive Care 43:193–198
Jäderling G, Bell M, Martling C-R et al (2013) ICU admittance by a rapid response team versus conventional admittance, characteristics, and outcome. Crit Care Med 41:725–731. doi:10.1097/CCM.0b013e3182711b94
Amaral ACKB, Fowler RA, Pinto R et al (2016) Patient and organizational factors associated with delays in antimicrobial therapy for septic shock. Crit Care Med 44:2145–2153. doi:10.1097/CCM.0000000000001868
Ferrer R, Martin-Loeches I, Phillips G et al (2014) Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med 42:1749–1755. doi:10.1097/CCM.0000000000000330
Leon AC, Demirtas H, Li C, Hedeker D (2013) Subject-level matching for imbalance in cluster randomized trials with a small number of clusters. Pharm Stat 12:268–274. doi:10.1002/pst.1580
Acknowledgements
MEDUSA contributing sites and site investigators, alphabetical by site
University Hospital RWTH Aachen, Department of Intensive Care Medicine: Gernot Marx, Achim Schindler, Tobias Schürholz; Ilm-Kreis-Kliniken Arnstadt, Department of Anesthesiology and Intensive Care Medicine: Heike Schlegel-Höfner, Gunther Lehmann, Annett Sander, Steffen Friese, Christian Scholz; Helios Hospital Aue, Department of Anesthesiology and Intensive Care Medicine: Pia Fischer; Zentralklinik Bad Berka GmbH, Department of Anaesthesia and Intensive Care Medicine: Christina Fuchs, Lutz Becher, Norbert Salewsky, Torsten Schreiber; Charité Berlin, Department of Anesthesiology and Operative Intensive Care Medicine: Anton Goldmann, Didier Keh, Katrin Schmid; Hufeland-Klinikum Bad Langensalza, Department of Anesthesiology and Intensive Care Medicine: Winfried Menning, Renate Steuckart; Bundeswehrkrankenhaus Berlin, Department of Anesthesiology and Intensive Care Medicine: Robert Barz, Karin Dey, Meike Fahrenholz, Martin Müller; Vivantes Klinikum Neukölln-Berlin, Department of Anesthesiology, Surgical Intensive Care Medicine, and Pain Therapy: Herwig Gerlach, Susanne Toussaint; Helios Hospital Berlin-Buch, Department of Intensive Care Medicine: Jörg Brederlau; Ev. Hospital Bielefeld, Department of Anesthesiology, Emergency and Intensive Care Medicine, and Pain Therapy: Friedhelm Bach, Dirk Buschmann, Ingo Gummelt, J. Hoeschen, Marion Klaproth, Ina Vedder; HELIOS-Hospital St. Josefs-Hospital Bochum-Linden, Department of Anesthesiology: Ulrike Bachmann-Holdau; St. Georg Hospital Eisenach, Department of Anesthesiology and Intensive Care Medicine: Jürgen Eiche, Rolf Hauschild; Hospital Rudolf Elle, Eisenberg, Department of Anesthesiology and Intensive Care Medicine: Martina Lange, Davia Herrmann-Karbaum; Helios-Hospital Emil-von Behring, Department of Interdisciplinary Intensive Care and Emergency Medicine: Annette Lubasch, Marcus Rücker; Helios-Hospital Erfurt, Department of Anesthesiology and Intensive Care Medicine: Christian Icke, Alexander Lucht, Andreas Meier-Hellmann, Jan Wagner; Catholic Hospital St. Johann Nepomuk Erfurt, Department of Anesthesiology and Intensive Care Medicine: Olaf Arnold, Steffen Kästner, Tobias Clausen; Hospital Friedberg, Department of Internal Medicine: Michael Sternkopf, Robert Voswinckel; SRH Waldklinikum Gera, Department of Anesthesiology and Intensive Care Medicine: T. Benndorf, Christel Eiserloh, Gerhard Kuhnle, Mathias Koch; University Hospital Greifswald, Department of Anesthesiology and Intensive Care Medicine: Manuela Gerber, Matthias Gründling, Liane Guderian, Sven-Olaf Kuhn, Christian Scheer; Hospital Ilmenau; Department of Anesthesiology and Intensive Care Medicine: Gerd Scheiber; Jena University Hospital, Center for Sepsis Control & Care/Department of Anesthesiology and Intensive Care Medicine: Frank Bloos, Susann Christink, Martina Kortegast, Claudia Matthäus-Krämer, Marcel Mücke, Bernhard Poidinger, Hendrik-Rüddel, Ulrike Redlich, Daniel Schwarzkopf, Daniel Thomas-Rüddel, Christel Volkmer; University Hospital Kiel, Department of Anesthesiology and Intensive Care Medicine: Stefanie D’Aria, Thees Lemke, Birgit Michaelsen, Dirk Schädler, Nina Schulz-Ruhtenberg, Norbert Weiler; Hospital Landshut-Achdorf, Department of Anesthesiology and Surgical Intensive Care Medicine: Martin Anetseder, Zoran Textor; University Hospital Leipzig, Department of Anesthesiology and Intensive Care Medicine: Udo Kaisers, Philipp Simon; Hospital Meiningen, Department of Intensive Care and Emergency Medicine: Georg Braun, Nicole Jensen, Werner Gegenfurtner, Alexander Meinhardt, Robert Schmitt, Andrea Teichert; Saale-Unstrut-Hospital Naumburg, Department of Anesthesiology and Intensive Care Medicine: Klaus-Dieter Becker; Hospital Oldenburg, Department of Anesthesiology, Intensive Care Medicine; Emergency Medicine, and Pain Therapy: Anja Diers, Florian Jelschen, Andreas Weyland; Thüringen-Klinik Pößneck, Department of Anesthesiology and Intensive Care Medicine: Frieder Knebel, Thomas Kupfer; Asklepios Hospital Radeberg, Department of Intensive Care and Emergency Medicine: Rüdinger Sinz; Thüringen-Kliniken Saalfeld, Department of Anesthesiology, Intensive Care Medicine, and Pain Therapy: Petra Bautz, Annemarie Fischer; Ev. Jung-Stilling Hospital Siegen, Department of Anesthesiology, Intensive Care, and Emergency Medicine: Armin Seibel, Christoph Fleischhacker; University Hospital Tübingen; Department of Anesthesiology: Helene Häberle, Philipp Henn, Friederike Mezger, Peter Rosenberger; University Hospital Tübingen; Dept of Internal Medicine: Reimer Riessen, Silvia Ziegler; University Hospital Medical School Ulm, Clinic of Anaesthesiology: Eberhard Barth, Hendrik Bracht, I. Heymann, A. Hinder, R. Sens, Manfred Weiss; Hufeland Hospital Weimar; Department of Anesthesiology and Intensive Care Medicine: Christof Lascho, Henriette Micke, Falk Schmidt; Helios Hospital Wuppertal. Department of Intensive Care Medicine: Stefanie Schilling, Gabriele Wöbker.
Clinical data collection and management
Matthias Löbe, Frank Meineke, Christine Pausch, Christoph Engel (Institute of Medical Informatics, Statistics and Epidemiology, University of Leipzig); statistical support for protocol development: Heike Hoyer (Institute for Medical Statistics, Information, and Documentation: Jena University Hospital); statistical support for implementation of the multiple imputation method: S. Grund, A. Robitzsch (Leibnitz Institute for Science and Mathematics Education, University Kiel).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr. Bloos reported receiving lecture honoraria from biosyn, Gilead, and CSL Behring. Dr. Harbarth reported receiving personal fees from Johnson & Johnson and Novartis and grants from Pfizer and BioMerieux. Dr. Levy reported receiving grants and personal fees from ImmuneExpress. Prof. Reinhart reported receiving grants and non-financial support from biosyn AG and Thermofischer/BRAHMS, being a shareholder of InflaRx Jena, and receiving personal fees from Adrenomed. Dr. Schuerholz reported grants and personal fees from Astellas Pharma, grants from B Braun Melsungen and Köhler Chemie, personal fees from Bayer Healthcare. Dr. Weyland reported receiving recruitment fees from Jena University Hospital for another trial.
Dr. Bach, Dr. Dey, Dr. Engel, Dr. Gerlach, Dr. Gründling, Dr. Häberle, Dr. Kaisers, Dr. Marshall, C. Matthäus-Krämer, Dr. Meier-Hellmann, Dr. Poidinger, Dr. Riessen, Dr. Rüddel, Dr. Scheer, Dr. Schreiber, D. Schwarzkopf, Dr. Simon, Dr. Thomas-Rüddel, Dr. Weiler, Dr. Weiss, and Dr. Woebker reported no conflicts of interest.
Funding/support
The study was funded by the German Federal Ministry of Education and Research via the integrated research and treatment center “Center for Sepsis Control and Care” (FKZ 01EO1002).
Additional information
Members of the MEDUSA study group are listed in the "Acknowledgements".
Take-home message: This first cluster-randomized controlled multicenter clinical trial addressing the effect of time to empirical antimicrobial therapy on 28-day mortality was unable to reduce time to antimicrobial therapy in the intervention group. Although the intervention itself did not affect survival, time to first antimicrobial therapy and time to source control were both associated with mortality.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bloos, F., Rüddel, H., Thomas-Rüddel, D. et al. Effect of a multifaceted educational intervention for anti-infectious measures on sepsis mortality: a cluster randomized trial. Intensive Care Med 43, 1602–1612 (2017). https://doi.org/10.1007/s00134-017-4782-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-017-4782-4