Abstract
Purpose
Echocardiography is frequently used in the hemodynamic evaluation of critically ill patients, but inaccurate measurements may lead to wrong clinical decisions. The aim of our systematic review was to investigate the interchangeability of echocardiography with thermodilution technique in measuring cardiac output and its changes.
Methods
In August 2015 we systematically searched electronic databases and included studies investigating the echocardiographic measurement of cardiac output compared with thermodilution technique using the Bland–Altman method. Two authors independently reviewed the studies and extracted data on type of measurements, clinical setting and characteristics, and those of the Bland–Altman and trending ability analyses.
Results
We identified 13,834 citations and included 24 studies in the final analysis. The median number of participants was 32 (range 8–65). Most of the studies assessed left-sided heart structures and the majority had small bias, wide limits of agreement, and high percentage error between echocardiography and thermodilution. In only two of the 24 studies the precision of each technique (echocardiography and thermodilution) was assessed before comparing them. In the single study evaluating trending ability using valid methodology, agreement was observed between echocardiography and thermodilution in detecting the directional changes in cardiac output, but the magnitude of changes varied considerably.
Conclusions
The majority of studies comparing echocardiography with thermodilution were difficult to interpret, but current evidence does not support interchangeability between these techniques in measuring cardiac output. The techniques may be interchangeable in tracking directional changes in cardiac output, but this has to be confirmed in large high-quality studies.
Similar content being viewed by others
Introduction
Measurements of cardiac output (CO) are frequently done in surgical and critically ill patients as part of optimization strategies [1]. Since the 1970s the use of pulmonary artery or Swan–Ganz catheter together with intermittent thermodilution technique based on the Stewart–Hamilton equation has been considered the clinical standard in measurements of CO. It has been extensively investigated in many different clinical settings and has demonstrated a precision of less than 10 % when used in scientific investigations [2–6]. On the other hand, the technique has its limitations, including variability in serial measurements of CO and rare, but potentially serious, complications [7, 8].
Two-dimensional echocardiography is a non-invasive [transthoracic (TTE)] or semi-invasive [transesophageal (TEE)] method for the assessment cardiac anatomy and function [9–12]. When Doppler technique or the volumetric method (difference of end-systolic and end-diastolic volume) is added, it is possible to measure stroke volume (SV) and CO. The anatomic sites for measuring velocity likely affect CO measurement and in the guidelines from the American Society of Echocardiography it is recommended that the left ventricular outflow tract (LVOT) is used for measurements of CO [13].
It is complex to validate a new technique for the measurement of CO. The new method has to be compared with an accepted clinical standard technique and the results analyzed using the Bland–Altman method, in which the agreement is evaluated between the new and the established technique [14, 15]. As no true gold standard exists for CO measurement the Bland–Altman method applies the mean of both the new and the reference technique. One plots the means of all the differences between the techniques (bias) and plots the variance, being ±1.96 standard deviations (SD) around the bias, also called limits of agreement (LOA) [14]. However, the acceptable LOA varies considerably, depending on the precision of the methods tested. Therefore, in any study attempting to validate a new method, it is imperative to determine the precision of the methods tested. Without knowledge of the precision of each method, it is impossible to determine the acceptable LOA and make a sound and unbiased conclusion of the interchangeability between the two methods.
Erroneous CO measurements could potentially lead to wrong clinical decisions including inappropriate use of fluid or inotropic drugs. This may potentially harm patients. As echocardiography is widely used in the clinical setting and promoted by experts [16], it is imperative to ensure that its validation is based on high-quality studies using the Bland–Altman analysis when assessing the agreement. The aim of this systematic review was to examine the evidence of echocardiography for measurements of CO. The thermodilution technique was chosen as clinical standard, and data regarding the reproducibility (precision) of each method and agreement and trending ability between them were obtained.
Methods
We used the methodology of the PRISMA guidelines for the conduct of systematic reviews [17]. The objective was a systematic review of the literature investigating echocardiographic measurements of CO compared with thermodilution technique with use of the Bland–Altman method. Potentially eligible studies were those assessing echocardiography-derived measurements of CO with thermodilution technique as the reference method in adult hospitalized patients. We performed an electronic literature search in Pubmed, Embase, and the Cochrane Library. The following search strategy was used to identify relevant studies:
-
1.
echocard* OR echo OR cardiac ultrasound OR TTE OR TEE OR transtho* OR transeso*.
-
2.
CO OR cardiac output OR SV OR stroke volume OR hemodynamic OR monitoring.
-
3.
#1 AND #2.
Study selection
The inclusion criteria were (1) hospitalized patients, (2) prospective clinical studies, systematic reviews, or meta-analysis, (3) adults (age >17 years), (4) the use of echocardiography-derived data to assess SV or CO, (5) the use of thermodilution technique as the reference technique, and (6) the use of the Bland–Altman method to compare them. Two authors (M.W. and R.R.J.) independently screened the articles for inclusion. All potentially relevant studies were reviewed in full text. If a study did not fulfill all inclusion criteria, it was excluded. The search was performed March 2014 and updated in August 2015.
We did not have predefined limitations regarding year of publication, language, populations of hospitalized patients, or predefined outcomes. Studies only published in abstract form were excluded if data allowing sufficient data assessment could not be obtained. Any disagreements with respect to inclusion/exclusion of studies were resolved with the co-authors (H.M.S., A.P.).
Data extraction
Two authors (M.W. and R.R.J.) extracted relevant data from included articles. The extracted data included the year of publication, patient population, number of patients, use of mechanical ventilation, ventilator mode, heart rhythm, type of monitor devices, echocardiographic view(s), the presence of valvular heart diseases, and echocardiographic- and thermodilution-derived data.
Because the different ways of measuring CO by echocardiography may not be interchangeable, we registered the method used (imaging performed from two-dimensional echocardiography combined with Doppler or volumetric technique). In the Doppler technique, CO is estimated by multiplying the velocity of time integral (VTi) of the blood flow by the cross-sectional area of an anatomic site (e.g., valve). In the volumetric method, SV is assesses by calculating the difference between end-systolic and end-diastolic volume [13, 18]. If more than one site was used, we obtained data from all the sites that were compared with thermodilution technique and analyzed using the Bland–Altman method.
We extracted available data from the Bland–Altman analyses, including the precision of each technique, bias, LOA, percentage error (PE), and trending ability between the two techniques. The PE was defined as 1.96 SD divided by the mean CO of the two methods. If PE was not reported, we a posteriori calculated PE if the data were available.
Results
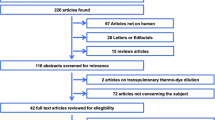
The search resulted in 13,834 unique citations; 13,720 citations were excluded on the basis of the title. From the remaining 114 citations, 26 citations were excluded on the basis of the abstract and 88 articles were evaluated in full text. Full-text studies were excluded if the Bland–Altman method was not used, thermodilution was not used, animals were studied, or the research question was irrelevant (not comparative CO measurements studies, studies evaluating fluid responsiveness, no use of echocardiography, and echocardiographic assessment of cardiac anatomy only) (Fig. 1). Twenty-four studies were included.
Characteristics of included studies
Of the 24 included studies, CO measurements was done with TEE in 19 studies [5, 19–36] and with TTE in five studies [37–41]. The median number of included patients was 32 (range 8–65). The majority of studies were performed in cardiac and intensive care unit (ICU) populations. Fifteen of the TEE studies were performed during elective cardiac surgery under hemodynamically stable conditions and in patients undergoing mechanical ventilation [5, 19–26, 29, 31–33, 35, 36], while eight studies were performed in ICU populations including septic shock, acute myocardial infarction, pulmonary hypertension, subarachnoid hemorrhage, and after liver transplantation [27, 28, 30, 37–41]. In none of the included studies were details given regarding ventilation mode or settings.
The TEE studies most frequently assessed the LVOT [5, 26, 27, 32–35], the aortic valve [23, 31, 33, 34, 36], and the mitral valve [19, 21, 22, 30, 34]. In the TTE studies, LVOT [38, 40, 41] and the aortic valve [37, 39] were the most often assessed.
The number of heartbeats used to calculate CO varied between 3 and 5 in the majority of studies [5, 19–22, 27, 29, 30, 32–38, 40]. The CO measurements with thermodilution were the mean of three measurements in most of the studies [20, 22–29, 31–37, 39, 41], while 4–5 measurements were used in four studies [5, 21, 30, 38].
Measurements of CO were performed in patients with sinus rhythm in the main part of the studies [5, 19–23, 25–27, 29–36, 39, 40]; in five studies it was not described if patients with arrhythmia were included [24, 28, 37, 38, 41]. The majority of studies excluded patients with significant valvular diseases; however, mitral regurgitation [19, 22, 26, 38, 40] and tricuspid regurgitation [21, 26, 29, 34, 40] were observed in some studies.
Assessment of changes in cardiac output were mainly performed at fixed time periods intra- and postoperatively [5, 19, 20, 22–24, 29, 31–37]. Other studies induced changes in CO by fluid loading or vasoactive drugs [5, 21, 28, 30, 36, 37]. In one study preload was corrected prior to CO measurements; patients received fluid pre- and postoperatively to increase cardiac filling pressure (defined by pulmonary artery diastolic pressure and right atrial pressure). Subsequently, the groups were randomized to receive 5 % albumin to maintain (normovolemic) or increase cardiac filling pressure (hypervolemic) before follow-up CO measurements [37].
In the majority of studies the investigations were blinded and off-line analyses were performed without randomization of the sequence of measurement [5, 19, 20, 23–27, 29, 30, 32–37, 39]; in three studies a random allocation process was used [19, 34, 36].
Echocardiographic assessment of cardiac output
Overall, the majority of studies showed small bias, wide LOA, and high PE between CO measured by echocardiography and thermodilution (Table 1; Figs. 2, 3). Percentage error was specified in four studies, and we could calculate PE a posteriori in an additional 11 studies. The PEs (specified and calculated a posteriori) ranged from approximately 16 to 69 % for LVOT [5, 26, 27, 31–35, 41], approximately 16 to 43 % for aortic valve [23, 33, 34, 36, 39], approximately 41 to 50 % for mitral valve [21, 30, 34], and from approximately 11 to 61 % for the right ventricular outflow tract (RVOT), pulmonary artery (PA), or pulmonary valve (PV) [29, 33, 34].
Forest plot including bias, limits of agreement, and percentage error (PE) of data from studies comparing cardiac output as assessed by echocardiography vs. thermodilution. The data were derived from the Bland–Altman analysis of the included studies. Only studies reporting both bias and limits of agreement are represented in the forest plot. Percentage errors are given if these were reported or could be calculated from the reported data. If not, not assessable (NA) is given
Assessment of cardiac output by echocardiography using Doppler. Estimation of stroke volume is done by the measuring of a diameter of a cardiac structure to assess the cross-sectional area (CSA) and multiplying it by the velocity time integral (VTI) obtained by the Doppler. The cardiac output is calculated by multiplying stroke volume by heart rate. aA ascending aorta, CO cardiac output, CSA cross-sectional area, d diameter, HR heart rate, LA left atrium, LV left ventricle, RV right ventricle, SV stroke volume, VTI velocity time integral
In only two studies [5, 28] were the precision assessed of both echocardiographic CO and thermodilution CO. Both studies were done using TEE and volumetric assessments were done in one [28]; in the other study Doppler was used at the LVOT [5]. The precision of thermodilution was 8 and 4.8 % in the two studies [5, 28]; that of TEE was 8.6 % (LOA ± 4.3 l/min) [28] and 16 % (±1.51 l/min) [5]. In these two studies the PE between CO measured by echocardiography and thermodilution was 53 [28] and 39 % [5].
Left ventricular outflow tract
In 11 studies the LVOT was used to measure CO by Doppler (Table 1); most of these had bias less than 0.5 l/min and LOA less than ±1.5 l/min, but PE was over 20 % in the majority of studies [26, 32–35].
The seven TEE studies measuring CO at the LVOT (Table 1) showed a bias ranging from −0.42 to 3.39 l/min and LOA varying from ±0.86 to ±1.51 l/min [5, 26, 27, 32–35]. Percentage error ranged from 16 to 48 %.
The four TTE studies measuring CO at the LVOT (Table 1) showed bias ranging from −0.75 to 0.4 l/min and LOA varying from ±0.83 to ±2.87 l/min [37, 38, 40, 41]. The only available PE from TTE-derived LVOT was 69 % [41].
Aortic valve
In six studies the aortic valve was used to measure CO (Table 1); most of the studies had bias below 0.5 l/min and LOA around ±1 l/min. The PE ranged from 16 to 43 % for both the TEE and TTE studies [23, 33, 34, 36, 39].
The five TEE studies measuring CO at the aortic valve showed bias ranging from −0.21 to 0.45 l/min [23, 31, 33, 34, 36] and LOA varying from ±0.83 to ±1.93 l/min. The PE of the TEE studies ranged from 19 to 43 % [23, 31, 33, 34, 36].
The one TTE study measuring CO at the aortic valve showed bias of −0.6 l/min and LOA ±1.2 l/min with PE of 16 % [39].
Mitral valve
In five studies the mitral valve was used to measure CO. All were done using TEE and bias varied from −0.86 to 4.29 l/min with LOA over 2 l/min in the majority of studies. The PE ranged from 41 to 50 % [19, 21, 22, 30, 34].
Right-sided heart structures and vessels
In six studies CO was measured using echocardiographic sites of the right-sided chambers and vessels of the heart, including the RVOT, the pulmonary artery, or the pulmonary valve (Table 1). In all studies TEE was used and bias ranged from −0.01 to 1.96 l/min, LOA from ±0.45 to ±2 l/min [19, 20, 25, 29, 33, 34], and PE from 11 to 61 % [29, 33, 34].
Volumetric assessment of cardiac output
In three studies of TEE [24, 25, 28] the measurement of CO was performed by the use of the volumetric method. One study assessed the precision of both TEE (9 %) and thermodilution (5 %); the bias between TEE and thermodilution was −0.5 l/min, LOA over ±4 l/min, and PE 53 % [28]. The studies evaluating the left ventricular areas found bias ranging from −0.2 to 0.47 and LOA between ±2.6 to 4.34 l/min [24, 25]. Percentage errors were not assessable for these two studies.
Trending ability
In 11 studies trending ability was investigated; in 10 studies TEE was used [5, 19, 20, 23, 24, 28–31, 35] and in one TTE was used [40]. In the majority of studies correlation analysis or receiver operating characteristic curves were used to assess changes in CO between echocardiography and thermodilution technique. In five studies controlled therapeutic interventions were performed to induce changes in CO to investigate trending ability [5, 21, 28, 30, 36].
In only three studies trending ability was evaluated by four-quadrant plot including concordance analysis or polar plot [5, 23, 31], but in two of these no therapeutic intervention was performed to assess trending ability [23, 31].
Both the precision of the two techniques and trending ability were evaluated in only one study [5]. This study showed concordance between echocardiography (TEE using Doppler of LVOT) and thermodilution regarding the ability to detect serial changes in CO. However, the magnitude of changes in CO varied considerably. Furthermore, the polar plot showed a radial degree of 53.5°, corresponding to poor trending ability despite an acceptable precision of each of the techniques [5]. The study by Perrino et al. [31] showed comparable findings, but the precision of both methods was not assessed [31]. In this study, Doppler technique at the aortic valve detected directional changes in 97 % of the cases, but tended to underestimate the magnitude of changes in CO compared with thermodilution [31].
Overall, in the three TEE studies where four-quadrant or polar plot was used, agreement was observed between echocardiography and thermodilution in tracking directional changes in CO [5, 23, 31].
None of the TTE studies assessed the trending ability with use of four-quadrant plot or polar plot. In one TTE study changes in CO were evaluated but only with the use of correlation analysis [40].
Discussion
In this systematic review we included 24 studies comparing echocardiography with thermodilution as the clinical standard technique in measuring CO, but only two studies had a design that allowed a fully unbiased comparison of the two techniques. The PE was 39 and 53 % between CO measured by echocardiography and thermodilution in these two studies [5, 28]. In the majority of the remaining studies, PE was above 20 % [21, 26, 30–36, 40, 41]. Only one study had a design that allowed a fully unbiased comparison of trending ability between echocardiography and thermodilution, and this showed agreement between the two techniques in the directional changes in CO [5]. This observation was supported by those of two other studies with analyses allowing reasonable comparison regarding directional changes in CO [23, 31].
The LVOT and the aortic valve are generally considered to be the most accurate echocardiographic sites for the measurement of SV and CO, because these are easily visualized and have low flow turbulence under normal physiological conditions [13]. This notion was supported in our results. LVOT and the aortic valve appeared to be the echo sites with the lowest bias; however, LOA and PE were still high. The accuracy may be affected by aortic regurgitation and obstruction of the LVOT. This may increase flow turbulence and changing blood flow, which will affect the accuracy of VTi measurements. However, these conditions were not recognized in the populations included in our review.
Imprecision in echocardiography CO measurements may be induced by patient, technical, or operator factors [42, 43]. Arrhythmias, low sedation levels, and changes in ventilator pressures and tidal volumes may affect the measurement of CO [44]. Furthermore, the assumption of laminar flow, constant cross-sectional area, and angle dependency of the probe may contribute to erroneous measurements. It has been shown by Hansen et al. [45, 46] that flow estimates done on complex in vivo blood flow, such as cardiac flow, is inaccurate when done with two-dimensional ultrasound methods. The main bias is the assumption of axisymmetrical and parabolic flow profiles, which is not met in in vivo vessel geometry. Thus, the lacking third dimension of in vivo volume flow estimation is likely the most important confounder for a precise estimation. Depending on the approach used for volume flow estimation from either Vmax or Vmean obtained with TEE or TTE, assumptions of parabolic and axisymmetical flow patterns are necessary, and therefore these assumptions create major biases. Therefore, measurements of CO with Doppler ultrasound carry a high risk of inaccuracy in estimation of CO as the assumptions applied in the calculation do not apply in complex cardiac flow. However, this also gives a plausible explanation of the apparent ability of Doppler ultrasound to track directional changes in CO, but not the magnitude, as changes in VTi may be maintained across the complex flow.
The variability in CO measured by echocardiography may be minimized by the use of mean values of repeated measurements [13]. In a study by Dubrey and Falk [47] it was necessary to measure around 13 heartbeats in patients with atrial fibrillation to obtain mean values of CO that varied less than 2 % compared with 3–5 heartbeats in patients with sinus rhythm. The studies included in our review measured CO in hemodynamically stable populations with sinus rhythm measured in 3–5 heartbeats.
Like any other CO measurement device, the thermodilution methods have variations in accuracy and precision. Errors in measurement of CO may be induced by the pathophysiology of the patient, biological variation, and technical problems [48–51]. Thermodilution is sensitive to tricuspid regurgitation and intracardiac shunts, which may result in falsely low CO measurements because of the recycling of indicator fluid across the tricuspid valve [52, 53]. The frequency of tricuspid regurgitation may be higher in a mechanically ventilated as compared with spontaneously breathing patients. A study by Balik et al. [54] showed that a high degree of tricuspid regurgitation resulted in lowered accuracy of thermodilution and underestimated CO in mechanically ventilated patients [54]. This could potentially have affected our results; however, one might assume that in studies comparing thermodilution with echocardiography the presence of tricuspid regurgitation or significant intracardiac shunts would be described. We only identified a few studies including mechanically ventilated patients with mild to moderate tricuspid regurgitation or intracardiac shunt [21, 26, 29, 34, 40]. Unfortunately, ventilation settings in these studies were not described further. Thermodilution is also sensitive to changes in injectate volume, fluid status, body temperature, and volume of injected fluid bolus [48, 55], which all may be sources of measurement error and variability. The inherent error was reduced in the studies we included by averaging serial measurements of CO for both thermodilution and echocardiography. Pulmonary artery catheter thermodilution can either be measured as intermittent or continuous measurement of CO.
Intermittent bolus thermodilution has been thoroughly investigated in many clinical settings and has been performed with a precision below 10 % at least in scientific investigations [2, 5, 6, 56] and continuous thermodilution has shown equally good reproducibility [57]. The difference between the two methods is the sampling time: intermittent bolus thermodilution is most often performed as the mean of three consecutive measurements, and continuous thermodilution is an average value for CO measured over the previous 5–15 min [58, 59]. This makes the continuous method less sensitive for variations in stroke volume caused by tricuspid regurgitation but also less time-responsive when sudden changes in CO occur. In all the studies included in this review intermittent bolus thermodilution was used.
Knowing that the precision of the methods varies makes it even more important to determine the precision of the two techniques before comparing them [60]. The majority of studies included in our review did not assess the precision of the two techniques compared (echocardiography and thermodilution), so it is not possible to evaluate the acceptable agreement between them or to determine the interchangeability [14]. Some of the included studies accepted echocardiographic CO based on the often-used PE cutoff value of ±30 % proposed by Critchley and Critchley [61]. However, the conclusions of these studies are less valid, because the precision of the reference technique could be more or less than that assumed. Furthermore, we observed wide variations in the identified or calculated PE from the included studies. It is possible that PE varies depending on the CO value (e.g., lower CO may have a higher PE than higher CO), which could result in erroneous interpretation.
It is important that changes in SV or CO can be measured because clinicians not only work with single point values but also with the changes, in particular following interventions. Using the Bland–Altman method the precision and accuracy is assessed, but the trending ability is not. Currently, the most accepted analytic approach in evaluating trending ability is the four-quadrant plot with concordance analysis or the polar plot [62, 63]. In only three studies in our review were trending ability evaluated and analyzed with the use of four-quadrant plot or polar plot [5, 23, 31]. The included studies showed that the directional changes between echocardiography and thermodilution were in agreement. However, the magnitude between the techniques varied and echocardiography was insensitive in detecting small changes in CO measured.
The strengths of this systematic review include the extensive literature search done systematically in multiple databases without language restriction of identified articles, and two authors structured the inclusions and exclusions independently. However, the search string did only include English words and we cannot be sure that our search strategy was adequate and identified all relevant studies.
The limitations of this study were the heterogeneity of the included studies, the low sample size, the sparse clinical data, and insufficient use of the Bland–Altman analysis. We did not perform meta-analyses of the results because of the limited amount of valid data. Furthermore, the results cannot be used to assess the effects of echocardiography-based strategies on outcome. Our aim was restricted to the validation of echocardiography in measurements of CO compared to thermodilution as the clinical standard. However, the overall assessment of hemodynamics using echocardiography is complex and the extensive literature research in this field was beyond the scope of our review.
Ideally new hemodynamic tools should perform with short time responsiveness, accurate amplitude responsiveness, and the ability to track directional changes in CO [64]. For this echocardiography may hold some promise, but it has to be confirmed in larger high-quality studies. Until then we suggest that clinicians do not make treatment decisions based on CO measured by echocardiography alone. Also it may be that VTi should be measured and used clinically rather than SV or CO, because it is less likely affected by the assumptions of laminar flow and constant cross-sectional area as described above. When evaluating echocardiography it is important to bear in mind that it provides valuable information about the cardiac anatomy, cardiac function, valve pathology, and imaging of blood flow and assessment of systolic and diastolic function of the heart [49, 65, 66]. For assessments of these quantities and qualities echocardiography is highly valuable in critically ill patients.
Conclusions
We systematically evaluated the interchangeability of echocardiography with thermodilution in assessing CO and its changes. However, methodologically proper evaluation with estimation of the precision of each technique before comparing them was only performed in few studies. The small sample size, heterogeneity of the studies, and inadequate statistical assessment do not allow us to make any definitive statement, but current evidence does not support interchangeability between these techniques in measuring cardiac output. Thermodilution and echocardiography may be interchangeable in tracking directional changes in cardiac output, but this has to be confirmed in large high-quality studies.
References
Rhodes A, Cecconi M, Hamilton M, Poloniecki J, Woods J, Boyd O, Bennett D, Grounds RM (2010) Goal-directed therapy in high-risk surgical patients: a 15-year follow-up study. Intensive Care Med 36:1327–1332
Nilsson LB, Nilsson JC, Skovgaard LT, Berthelsen PG (2004) Thermodilution cardiac output—are three injections enough? Acta Anaesthesiol Scand 48:1322–1327
Berthelsen PG, Eldrup N, Nilsson LB, Rasmussen JP (2002) Thermodilution cardiac output. Cold vs. room temperature injectate and the importance of measuring the injectate temperature in the right atrium. Acta Anaesthesiol Scand 46:1103–1110
Monnet X, Persichini R, Ktari M, Jozwiak M, Richard C, Teboul JL (2011) Precision of the transpulmonary thermodilution measurements. Crit Care 15:R204
Moller-Sorensen H, Graeser K, Hansen KL, Zemtsovski M, Sander EM, Nilsson JC (2014) Measurements of cardiac output obtained with transesophageal echocardiography and pulmonary artery thermodilution are not interchangeable. Acta Anaesthesiol Scand 58:80–88
Moller-Sorensen H, Hansen KL, Ostergaard M, Andersen LW, Moller K (2012) Lack of agreement and trending ability of the endotracheal cardiac output monitor compared with thermodilution. Acta Anaesthesiol Scand 56:433–440
Polanczyk CA, Rohde LE, Goldman L, Cook EF, Thomas EJ, Marcantonio ER, Mangione CM, Lee TH (2001) Right heart catheterization and cardiac complications in patients undergoing noncardiac surgery: an observational study. JAMA 286:309–314
Sandham JD, Hull RD, Brant RF, Knox L, Pineo GF, Doig CJ, Laporta DP, Viner S, Passerini L, Devitt H, Kirby A, Jacka M (2003) A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med 348:5–14
Haji DL, Royse A, Royse CF (2013) Review article: clinical impact of non-cardiologist-performed transthoracic echocardiography in emergency medicine, intensive care medicine and anaesthesia. Emerg Med Australas 25:4–12
Romero-Bermejo FJ, Ruiz-Bailen M, Guerrero-De-Mier M, Lopez-Alvaro J (2011) Echocardiographic hemodynamic monitoring in the critically ill patient. Curr Cardiol Rev 7:146–156
Guarracino F, Baldassarri R (2009) Transesophageal echocardiography in the OR and ICU. Minerva Anestesiol 75:518–529
Figueiredo A, Germano N, Guedes P, Marcelino P (2011) The evolving concepts of haemodynamic support: from pulmonary artery catheter to echocardiography and theragnostics. Curr Cardiol Rev 7:136–145
Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA (2002) Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr 15:167–184
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Berthelsen PG, Nilsson LB (2006) Researcher bias and generalization of results in bias and limits of agreement analyses: a commentary based on the review of 50 Acta Anaesthesiologica Scandinavica papers using the Altman–Bland approach. Acta Anaesthesiol Scand 50:1111–1113
Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, Jaeschke R, Mebazaa A, Pinsky MR, Teboul JL, Vincent JL, Rhodes A (2014) Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 40:1795–1815
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Hahn RT, Abraham T, Adams MS, Bruce CJ, Glas KE, Lang RM, Reeves ST, Shanewise JS, Siu SC, Stewart W, Picard MH (2014) Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. Anesth Analg 118:21–68
Muhiudeen IA, Kuecherer HF, Lee E, Cahalan MK, Schiller NB (1991) Intraoperative estimation of cardiac output by transesophageal pulsed Doppler echocardiography. Anesthesiology 74:9–14
Savino JS, Troianos CA, Aukburg S, Weiss R, Reichek N (1991) Measurement of pulmonary blood flow with transesophageal two-dimensional and Doppler echocardiography. Anesthesiology 75:445–451
Shimamoto H, Kito H, Kawazoe K, Fujita T, Shimamoto Y (1992) Transoesophageal Doppler echocardiographic measurement of cardiac output by the mitral annulus method. Br Heart J 68:510–515
Ryan T, Page R, Bouchier-Hayes D, Cunningham AJ (1992) Transoesophageal pulsed wave Doppler measurement of cardiac output during major vascular surgery: comparison with the thermodilution technique. Br J Anaesth 69:101–104
Darmon PL, Hillel Z, Mogtader A, Mindich B, Thys D (1994) Cardiac output by transesophageal echocardiography using continuous-wave Doppler across the aortic valve. Anesthesiology 80:796–805
Pinto FJ, Siegel LC, Chenzbraun A, Schnittger I (1994) On-line estimation of cardiac output with a new automated border detection system using transesophageal echocardiography: a preliminary comparison with thermodilution. J Cardiothorac Vasc Anesth 8:625–630
Izzat MB, Regragui IA, Wilde P, Angelini GD, Bryan AJ (1994) Transesophageal echocardiographic measurements of cardiac output in cardiac surgical patients. Ann Thorac Surg 58:1486–1489
Feinberg MS, Hopkins WE, Davila-Roman VG, Barzilai B (1995) Multiplane transesophageal echocardiographic doppler imaging accurately determines cardiac output measurements in critically ill patients. Chest 107:769–773
Descorps-Declere A, Smail N, Vigue B, Duranteau J, Mimoz O, Edouard A, Samii K (1996) Transgastric, pulsed Doppler echocardiographic determination of cardiac output. Intensive Care Med 22:34–38
Axler O, Tousignant C, Thompson CR, Dall’ava-Santucci J, Phang PT, Russell JA, Walley KR (1996) Comparison of transesophageal echocardiographic, fick, and thermodilution cardiac output in critically ill patients. J Crit Care 11:109–116
Maslow A, Comunale ME, Haering JM, Watkins J (1996) Pulsed wave Doppler measurement of cardiac output from the right ventricular outflow tract. Anesth Analg 83:466–471
Estagnasie P, Djedaini K, Mier L, Coste F, Dreyfuss D (1997) Measurement of cardiac output by transesophageal echocardiography in mechanically ventilated patients. Comparison with thermodilution. Intensive Care Med 23:753–759
Perrino AC Jr, Harris SN, Luther MA (1998) Intraoperative determination of cardiac output using multiplane transesophageal echocardiography: a comparison to thermodilution. Anesthesiology 89:350–357
Poelaert J, Schmidt C, Van Aken H, Hinder F, Mollhoff T, Loick HM (1999) A comparison of transoesophageal echocardiographic Doppler across the aortic valve and the thermodilution technique for estimating cardiac output. Anaesthesia 54:128–136
Zhao X, Mashikian JS, Panzica P, Lerner A, Park KW, Comunale ME (2003) Comparison of thermodilution bolus cardiac output and Doppler cardiac output in the early post-cardiopulmonary bypass period. J Cardiothorac Vasc Anesth 17:193–198
Bettex DA, Hinselmann V, Hellermann JP, Jenni R, Schmid ER (2004) Transoesophageal echocardiography is unreliable for cardiac output assessment after cardiac surgery compared with thermodilution. Anaesthesia 59:1184–1192
Parra V, Fita G, Rovira I, Matute P, Gomar C, Pare C (2008) Transoesophageal echocardiography accurately detects cardiac output variation: a prospective comparison with thermodilution in cardiac surgery. Eur J Anaesthesiol 25:135–143
Missant C, Rex S, Wouters PF (2008) Accuracy of cardiac output measurements with pulse contour analysis (PulseCO) and Doppler echocardiography during off-pump coronary artery bypass grafting. Eur J Anaesthesiol 25:243–248
Mayer SA, Sherman D, Fink ME, Homma S, Solomon RA, Lennihan L, Beckford A, Klebanoff LM (1995) Noninvasive monitoring of cardiac output by Doppler echocardiography in patients treated with volume expansion after subarachnoid hemorrhage. Crit Care Med 23:1470–1474
McLean AS, Needham A, Stewart D, Parkin R (1997) Estimation of cardiac output by noninvasive echocardiographic techniques in the critically ill subject. Anaesth Intensive Care 25:250–254
Marcelino P, Germano N, Marum S, Fernandes AP, Ribeiro P, Lopes MG (2006) Haemodynamic parameters obtained by transthoracic echocardiography and Swan-Ganz catheter: a comparative study in liver transplant patients. Acta Med Port 19:197–205
Temporelli PL, Scapellato F, Eleuteri E, Imparato A, Giannuzzi P (2010) Doppler echocardiography in advanced systolic heart failure: a noninvasive alternative to Swan-Ganz catheter. Circ Heart Fail 3:387–394
Tian Z, Liu YT, Fang Q, Ni C, Chen TB, Fang LG, Gao P, Jiang XC, Li MT, Zeng XF (2011) Hemodynamic parameters obtained by transthoracic echocardiography and right heart catheterization: a comparative study in patients with pulmonary hypertension. Chin Med J 124:1796–1801
Huang SJ, McLean AS (2012) Appreciating the strengths and weaknesses of transthoracic echocardiography in hemodynamic assessments. Cardiol Res Pract 2012:894308
van den Berg PC, Grimbergen CA, Spaan JA, Pinsky MR (1997) Positive pressure inspiration differentially affects right and left ventricular outputs in postoperative cardiac surgery patients. J Crit Care 12:56–65
Pinsky MR (2007) Heart–lung interactions. Curr Opin Crit Care 13:528–531
Hansen KL, Udesen J, Thomsen C, Jensen JA, Nielsen MB (2009) In vivo validation of a blood vector velocity estimator with MR angiography. IEEE Trans Ultrason Ferroelectr Freq Control 56:91–100
Hansen KL, Moller-Sorensen H, Kjaergaard J, Jensen, MB, Lund, JT, Pedersen MM, Olesen JB, Jensen JA, Nielsen MB (2015) Vector flow imaging compared with conventional Doppler ultrasound and thermodilution for estimation of blood flow in the ascending aorta. Ultrason Imaging. doi:10.1177/0161734615620137
Dubrey SW, Falk RH (1997) Optimal number of beats for the Doppler measurement of cardiac output in atrial fibrillation. J Am Soc Echocardiogr 10:67–71
Nishikawa T, Dohi S (1993) Errors in the measurement of cardiac output by thermodilution. Can J Anaesth 40:142–153
Vincent JL, Rhodes A, Perel A, Martin GS, Della Rocca G, Vallet B, Pinsky MR, Hofer CK, Teboul JL, de Boode WP, Scolletta S, Vieillard-Baron A, De Backer D, Walley KR, Maggiorini M, Singer M (2011) Clinical review: update on hemodynamic monitoring—a consensus of 16. Crit Care 15:229
Rhodes A, Cusack RJ, Newman PJ, Grounds RM, Bennett ED (2002) A randomised, controlled trial of the pulmonary artery catheter in critically ill patients. Intensive Care Med 28:256–264
Jansen JR (1995) The thermodilution method for the clinical assessment of cardiac output. Intensive Care Med 21:691–697
Jullien T, Valtier B, Hongnat JM, Dubourg O, Bourdarias JP, Jardin F (1995) Incidence of tricuspid regurgitation and vena caval backward flow in mechanically ventilated patients. A color Doppler and contrast echocardiographic study. Chest 107:488–493
Artucio H, Hurtado J, Zimet L, de Paula J, Beron M (1997) PEEP-induced tricuspid regurgitation. Intensive Care Med 23:836–840
Balik M, Pachl J, Hendl J (2002) Effect of the degree of tricuspid regurgitation on cardiac output measurements by thermodilution. Intensive Care Med 28:1117–1121
Thiele RH, Bartels K, Gan TJ (2015) Cardiac output monitoring: a contemporary assessment and review. Crit Care Med 43:177–185
Ostergaard M, Nilsson LB, Nilsson JC, Rasmussen JP, Berthelsen PG (2005) Precision of bolus thermodilution cardiac output measurements in patients with atrial fibrillation. Acta Anaesthesiol Scand 49:366–372
Reuter DA, Huang C, Edrich T, Shernan SK, Eltzschig HK (2010) Cardiac output monitoring using indicator-dilution techniques: basics, limits, and perspectives. Anesth Analg 110:799–811
Leibowitz AB, Oropello JM (2007) The pulmonary artery catheter in anesthesia practice in 2007: an historical overview with emphasis on the past 6 years. Semin Cardiothorac Vasc Anesth 11:162–176
Lee AJ, Cohn JH, Ranasinghe JS (2011) Cardiac output assessed by invasive and minimally invasive techniques. Anesthesiol Res Pract 2011:475151
Cecconi M, Rhodes A, Poloniecki J, Della Rocca G, Grounds RM (2009) Bench-to-bedside review: the importance of the precision of the reference technique in method comparison studies—with specific reference to the measurement of cardiac output. Crit Care 13:201
Critchley LA, Critchley JA (1999) A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput 15:85–91
Critchley LA, Lee A, Ho AM (2010) A critical review of the ability of continuous cardiac output monitors to measure trends in cardiac output. Anesth Analg 111:1180–1192
Critchley LA, Yang XX, Lee A (2011) Assessment of trending ability of cardiac output monitors by polar plot methodology. J Cardiothorac Vasc Anesth 25:536–546
Squara P, Cecconi M, Rhodes A, Singer M, Chiche JD (2009) Tracking changes in cardiac output: methodological considerations for the validation of monitoring devices. Intensive Care Med 35:1801–1808
Geisen M, Spray D, Nicholas Fletcher S (2014) Echocardiography-based hemodynamic management in the cardiac surgical intensive care unit. J Cardiothorac Vasc Anesth 28:733–744
Huttemann E (2006) Transoesophageal echocardiography in critical care. Minerva Anestesiol 72:891–913
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None of the authors have potential conflicts of interest.
Funding
Department funds only.
Additional information
Take-home message: Echocardiography is not interchangeable with thermodilution technique in measuring cardiac output. There may be concordance between the techniques in detecting directional changes in cardiac output.
Rights and permissions
About this article
Cite this article
Wetterslev, M., Møller-Sørensen, H., Johansen, R.R. et al. Systematic review of cardiac output measurements by echocardiography vs. thermodilution: the techniques are not interchangeable. Intensive Care Med 42, 1223–1233 (2016). https://doi.org/10.1007/s00134-016-4258-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-016-4258-y