Abstract
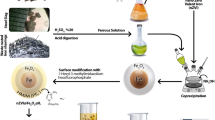
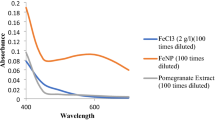
Alternative plant extracts were examined as raw materials for the synthesis of nZVI from ferric solutions. Four plants were selected for evaluation, i.e. Camellia sinensis (green tea, GT), Syzygium aromaticum (clove, CL), Mentha spicata (spearmint, SM) and Punica granatum (pomegranate, PG). Based on the results obtained, it was concluded that the reduction of Fe(III) with the herb extracts is not complete. Using the GT extract, approximately 28 mM of the initial 66 mM of Fe (42.4 %) are reduced to the elemental state Fe(0). The highest reduction of Fe(III), about 53 %, was achieved with PG and the lowest, only 15.6 %, with the SM extract. Additional batch experiments have been carried out to evaluate the effectiveness of nZVI, synthesized with GT, CL, SM and PG, for the removal of hexavalent chromium from a 0.96 mM solution. The highest reduction of Cr(VI) (96 %) was obtained using the nZVI suspension produced with PG juice. The other three nZVI suspensions, i.e. CL-nZVI, GT-nZVI, and SM-nZVI, had a comparable effectiveness corresponding to 70 % reduction of chromate.

Similar content being viewed by others
References
Carlsen H et al (2010) The total antioxidant content of more than 3,100 foods, beverages, spices, herbs and supplements used worldwide. Nutr J 9:3
Chrysochoou M, Ferreira D, Johnston CP (2010) Calcium polysulfide treatment of Cr contaminated soil. J Hazard Mater 179:650–657
Chrysochoou M, Johnston C, Dahal G (2012) A comparative evaluation of hexavalent chromium treatment in contaminated soil by calcium polysulfide and green-tea nanoscale zero-valent iron. J Hazard Mater 201–202:33–42
Crane RA, Scott TB (2012) Nanoscale zero-valent iron: future prospects for an emerging water treatment technology. J Hazard Mater 211–212:112–125
Elovitz MS, Fish W (1995) Redox Interactions of Cr(VI) and substituted phenols: products and mechanisms. Environ Sci Technol 29:1933–1943
Gheju M (2011) Hexavalent chromium reduction with zero valent iron (ZVI) in aquatic systems. Water Air Soil Pollut 222:103–148. doi:10.1007/s11270-011-0812-y
Hoag GE (2009) Degradation of bromothymol blue by ‘greener’ nano-scale zero-valent iron synthesized using tea polyphenols. J Mater Chem 19(45):8671–8677
Ignatiadis I, Morin D, Ragot C, Oudin JC, Dueso N (2005) Development and in situ implementation of a chemical process for immobilisation of the chromate contained in an industrial ground. In: Proceedings of 9th international conference on contaminated soil (ConSoil 2005), Bordeau, pp 2555–2564
Njagi EC, Huang H, Stafford L, Genuino H, Galindo H, Collins J, Hoag G, Suib S (2010) Biosynthesis of iron and silver nanoparticles at room temperature using aqueous sorghum bran extracts. Langmuir 27(1):264–271
Noubactep C (2008) A critical review on the mechanism of contaminant removal in Fe0–H2O systems. Environ Technol 29:909–920
Papassiopi N, Gaitanarou Z, Xenidis A (2012) Stabilizationn of chromium in the form of mixed Fe(III)–Cr(III) hydroxides. Fresenius Environ Bull (in press)
Perron N, Brumaghim J (2009) A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys 53:75–100
Phenrat T, Saleh N, Shirk K, Tilton R, Lowry G (2007) Aggregation and sedimentation of aqueous nanoscale zerovalent iron dispersions. Environ Sci Technol 41:284–290
Sanchez I, Stuber F, Font J, Fortuny A, Fabrgat A, Bengoa C (2007) Elimination of phenol and aromatic compounds by zero valent iron and EDTA at low temperature and atmospheric pressure. Chemosphere 68:338–344
Sass BM, Rai D (1987) Solubility of amorphous chromium(III)—iron(III) hydroxide solid solutions. Inorg Chem 26:2228–2232
Shahwan T, Abu Sirriah S, Nairat M, Boyacı E, Eroglu A, Scott T, Hallam K (2011) Green synthesis of iron nanoparticles and their application as a Fenton-like catalyst for the degradation of aqueous cationic and anionic dyes. Chem Eng J 172:258–266
USDA (2001) U.S. Department of the Army, Engineering and design precipitation/coagulation/flocculation. Manual No. 1110-1-4012, Army Corps of Engineers, Washington
Acknowledgments
The research of Mystrioti Christiana has been co-financed by the European Union (European Social Fund—ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF)—Research Funding Program: Heracleitus II. Investing in knowledge society through the European Social Fund. The rest authors would like to thank the LIFE+ CHARM project (LIFE10 ENV/GR/000601). The authors thank Prof. G. Demopoulos, McGill University, for his contribution to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mystrioti, C., Sparis, D., Papasiopi, N. et al. Assessment of Polyphenol Coated Nano Zero Valent Iron for Hexavalent Chromium Removal from Contaminated Waters. Bull Environ Contam Toxicol 94, 302–307 (2015). https://doi.org/10.1007/s00128-014-1442-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-014-1442-z