Abstract
Purpose
Global understanding of the epidemiological landscape of non-affective psychotic disorders (NAPD) is predominantly based on studies from high-income countries. We sought to systematically review and meta-analyse all incidence studies conducted in low and middle-income countries (LMICs).
Methods
We systematically searched four databases using terms for NAPD, incidence and LMICs. Citations were eligible for inclusion if: published between 1 January 1960 and 31 May 2022; wholly or partially conducted in an LMIC, and; containing data on NAPD incidence in the general adult population. Two independent raters assessed study quality according to previously published criteria. We conducted a narrative synthesis and random-effects meta-analyses where sufficient studies were available (N ≥ 5).
Results
We retrieved 11 421 records, of which 23 citations met inclusion criteria from 18 unique studies across 19 settings in 10 LMICs. Median study quality was 4 out of 7 (interquartile range: 3–6). The crude incidence of NAPD varied around 4.2 times, from 10.0 per 100,000 person-years (95% confidence interval [CI] 8.7–11.4) in Brazil to 42.0 (95%CI 32.2–54.8) in India, with marked heterogeneity in methodologies and rates. Our 60-year review highlights the dearth of robust evidence on the incidence of psychotic disorders in LMICs.
Conclusion
Without reliable, contemporary estimates of this fundamental cornerstone of population health, it is impossible to understand the true burden, distribution or causes of psychotic disorders in over 87% of the world’s population. A new, more equitable global mental health evidence base for NAPD is now urgently required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In little over 15 years, received wisdom on the epidemiology of psychotic disorders has changed from the view that they were uniformly distributed, to current understanding of a robust, replicable but varied distribution by person and place [1]. For example, systematic reviews and meta-analyses have confirmed that incidence rates of psychotic disorders are higher for younger men than women, peaking in late adolescence, before declining throughout adult life [2,3,4]. Rates are also consistently elevated for many ethnic minority groups in most settings where this has been studied [2,3,4,5], and appear higher in those exposed to more urban environments during the life course [6]. These findings potentially inform both aetiological understanding of psychotic disorders and the provision of timely and appropriate early intervention for psychosis.
Nonetheless, the epidemiological evidence base on which these findings are predicated arises almost exclusively from high-income countries [HICs], with only occasional, older exceptions [7,8,9,10]. This is problematic for several reasons: first, observed patterns of the distribution of psychotic disorders in one country or narrow range of countries may not generalise to other settings; second, alternate patterns of exposures and outcomes in other settings may refute, support or refine current aetiological understanding, and; finally, epistemological thinking restricted to a small set of countries may create hegemonic structures which fail to reveal the true underlying aetiology of psychotic disorders across different settings. In other words: what we think we know about non-affective psychotic disorders [NAPD] is mainly based on evidence from HICs, and this might not hold true universally.
A useful example is the study of the association between urbanicity and psychosis. Studies conducted in HICs frequently report higher incidence rates of NAPD in more urban populations [6], including associations with urban birth [11]. However, results from a cross-sectional survey of 42 Low and Middle Income Countries [LMICs] in the World Health Organization [WHO] World Health Survey [12], reported no consistent association between the prevalence of psychotic symptoms and contemporaneously-estimated rural or urban exposure. Although comparing such findings is difficult [13], recent evidence from a nationwide study in Chile [14] and multinational [15] incidence data from Europe and Brazil also found that regional deprivation was more strongly associated with the incidence of psychotic disorders than population density, usually considered a more direct marker of urbanicity; these studies suggest that different patterns of association between risk factors and psychosis may be present in different contexts. Unfortunately, evidence on the incidence of psychotic disorders—a central epidemiological cornerstone for understanding the burden, distribution, and aetiology of disease—from LMICs is scant. To address and quantify this issue, our objective was to systematically review the literature on the incidence of NAPDs in LMICs published between January 1960 and May 2022.
Methods
Search strategy and selection criteria
In this systematic review and meta-analysis we followed PRISMA guidelines (Supplemental Table 1), including protocol preregistration on PROSPERO (reference: CRD42020179678). We adapted a previous methodology we developed for global and national systematic reviews [2, 4], based on Cochrane systematic reviewing guidelines. Briefly, we systematically searched MedLine, PsycINFO, Web of Science, and Embase using a comprehensive search strategy (Supplemental Materials, Sect. 1) with application of the Cochrane filter for LMICs [16]. Citations were eligible for inclusion if they were:
-
published 1 January 1960—31 May 2022;
-
wholly or partially conducted in a LMIC (as defined by The World Bank [17]);
-
contained sufficient original data on, or to derive, the incidence of NAPD;
-
conducted in the general adult population (aged ~ 16–64).
Citations published between 1960 and 2001 and 2019–2022 were retrieved by searching the aforementioned databases. Citations published between 2002 and 2018 were identified from an existing open access database maintained by HEJ from our recent global review of the literature using an identical search strategy [4]. We also searched the references of included citations for potentially missed citations, as well as the resource databank of the Global Burden of Disease Study [18]. We placed no restriction on language of publication, study design or publication status, although grey literature was only identified via conference proceedings, author correspondence, and reference searching. One author (RJ-BB) carried out the searches, and three authors (RJ-BB, JBK, HEJ) screened citations at title stage. Two authors (HEJ, RJ-BB) independently and in duplicate screened citations at abstract and full-text review stages (Supplemental Materials, Sect. 2), with disagreements solved by consensus with JBK.
Data extraction
One author extracted data from included studies (RJ-BB), with consistency and accuracy checks performed by HEJ and JBK. Rate-level data about incidence, and meta-level data on study characteristics, time period and study quality were included.
Our primary outcome was the crude incidence of NAPD per 100,000 person-years, based on the diagnostic classification system used in each study (Supplemental Materials, Sect. 3). Under the International Classification of Diseases (10th revision) [ICD-10], this was defined as F20–29 and includes schizophrenia, schizotypal disorder, persistent delusional disorder, acute and transient psychotic disorders, induced psychotic disorders, schizoaffective disorders, other non-organic psychotic disorders and unspecified non-organic psychosis. We assumed sufficiently commonality across systems to permit comparison of rates. Though this was not an inclusion criterion, here available, we also extracted and reported incidence rates for: schizophrenia (F20); affective psychoses (F30.2, F31.2, F32.3, F33.3), and; all clinically relevant psychotic disorders (F20-33, F1X.5). We only included studies where a formal diagnosis of NAPD was made, so religious, alternative or traditional healers were only included if there was a cooperation with a medical care provider. We recorded the numerator (case) and denominator (population at-risk), rate, standard error and 95% confidence intervals [95%CI], where reported. Where available, we extracted incidence data by sex.
We also recorded meta-level data on study design, study quality, and time period. Study design was defined as first contact (with any healthcare provider for NAPD), first episode (of NAPD), first admission (to any psychiatric facility for NAPD), cohort or household surveys. Two independent raters (AG-V and LV) assessed study quality according to seven previously published criteria relevant to incidence studies of psychotic disorders [2, 4] (Supplemental Materials, Sect. 4). Discrepancies were resolved by consensus with HEJ and JBK. Time period was defined as the median year of the case ascertainment period. Where multiple citations reported data from the same sample, both were included in the systematic review, with a core citation defined based on the one containing the most detailed estimates of incidence for inclusion in any meta-analysis.
Data analysis
We conducted a narrative synthesis of the yield, reporting descriptive characteristics of included citations according to our meta-level variables, followed by synthesis of rates by continent, sex and study quality. Where there was sufficient data (n ≥ 5), we conducted random-effects meta-analysis (DerSimonian and Laird [19] method), since we anticipated high between-study heterogeneity [4]. Incidence rates were transformed onto the natural logarithmic scale alongside their corresponding standard errors (SEs). If no SE could be derived, we retained rates for narrative synthesis only. For assessments of differences in incidence by sex, we repeated this methodology for incidence rare ratios (IRRs) in men compared with women from available citations. Given the paucity of studies which reported standardized rates (i.e. for age and/or sex), we restricted meta-analyses to crude incidence comparisons only.
We tested heterogeneity using the Q-test and quantified this using the I2-statistic. In a change to the original protocol on PROSPERO, we did not analyse pooled estimates from meta-analyses because between-study heterogeneity was high (I2 ≥ 75%) [20]. We examined evidence of small study effects (including publication bias) by visual inspection of funnel plots (N ≥ 5) and formal testing using Egger’s test when sufficient estimates (N ≥ 10) were available.
Citations were managed in Mendeley (version 1.17.12) and extracted data were managed in an Excel spreadsheet. Meta-analyses were conducted in R using the ‘meta’ package [21] by one author (TD).
Role of the funding source and ethical considerations
Funders had no role in any aspect of this study including the decision to submit the paper for publication. No ethics approval was required for this study, as no original data was gathered.
Results
Characteristics of included studies
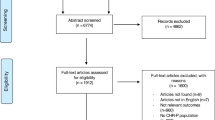
We retrieved 8 664 records after removal of duplicates, of which 23 met inclusion criteria (Fig. 1) [7,8,9,10, 15, 22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]. Four citations reported overlapping data from two centres (India, USSR) of the WHO “10-country study” [8, 9, 29, 30]; where relevant, we used data from Jablensky et al. [8] as our core citation (Supplemental Materials, Sect. 5). Two further citations [33, 34] provided overlapping incidence data from a study in Haidian District, Beijing, with Chen et al. [33] providing the core citation. Two studies provided overlapping incidence data from Ribeirão Preto (Brazil) [37] with Jongsma et al. [15] providing the core citation. Despite extensive searches, including contacting the authors of an earlier global systematic review of the incidence of schizophrenia [3], we were unable to obtain the full text of two core citations which met our inclusion criteria based on abstract information [31, 32]; we were able to include data from one of these [32] based on available data published in that review [3].
From 18 core citations [7, 8, 10, 15, 22,23,24,25,26,27,28, 31,32,33, 35, 36, 39], 15 (83.3%) had sufficient data available to include in meta-analyses [7, 8, 10, 15, 22,23,24,25,26,27,28, 33, 35, 39], with two studies not reporting sample sizes or standard errors alongside incidence rates [31, 32] and a further study providing standardised incidence rates only [36]. The median sample size from the remaining 15 studies was 320 cases (interquartile range: 70–809; range: 15 cases of schizophrenia in India [22] to 178 173 cases in Brazil [25]; Table 1). Core citations contributed original incidence data from 19 separate settings (Morgan et al. [39] and Jablensky et al. [8] each included data from two sites) in ten LMICs in South America (N = 8; 50.0%; Brazil [7, 15, 25, 36], Suriname [23, 27], Colombia [38] and Costa Rica [26]), Asia (N = 5; 35.7%; India [8, 22, 39], China [33, 35]), Africa (N = 3; 21.4%; Nigeria [28, 39], South Africa [24]), Europe (N = 3; 21.4%; Moscow, former USSR [8, 31, 32]), and North America (N = 1; 6.7%; Jamaica [10]). No studies were identified from LMICs in Australasia. Median year of case ascertainment was 1992 (IQR: 1979–2005), ranging from 1967 [25] to 2013 [15]. All core citations used a first contact or admission design, except for two household surveys in India [22] and China [33]. Only four citations (26.7%) reported age-sex [15, 23, 27, 39] (and in one study [15], age-sex-ethnicity) standardized rates.
Incidence of psychotic disorders
Six core citations (40.0%) included eight separate estimates for NAPD in five LMICs (Fig. 2a) [7, 8, 10, 15, 23, 27]. We observed a fourfold variation in crude incidence, from 10.0 cases per 100,000 person-years in Sao Paulo, Brazil (95%CI 8.7–11.4) [7] to 42.0 (95%CI 32.2–54.8) in rural Chandigarh in India [8]. Heterogeneity was high (I2: 97.3%, Q: 259.6; p < 0.01).
Schizophrenia was the most frequently studied diagnostic outcome with 13 unique rates reported from ten (55.6%) core citations from eight LMICs (Fig. 2b) [8, 10, 22, 25, 26, 28, 32, 33, 39]. Crude incidence varied approximately fivefold, from 9.0 cases per 100,000 person-years (95%CI 6.5–12.5) in India [8] to in 48.2 (95%CI 46.5–50.0) in Costa Rica [25]. Heterogeneity was high (I2: 99.3%; Q: 1600.8, p < 0.0001).
Four core citations (26.7%) reported the incidence of all clinically relevant psychotic disorders in Brazil, India, Nigeria and South Africa (Fig. 2c) [15, 23, 24, 39]. Rates varied from 15.8 per 100,000 person-years (95%CI 14.3–17.6) in Sao Paulo, Brazil, to 45.9 (95%CI 34.5–57.3) in Chengalpattu, India [39]. Heterogeneity was high (I2: 98.0%; Q: 99.9, p < 0.01).
Four core citations (26.7%) also reported incidence data for the affective psychoses from four settings [7, 10, 15, 25], three in Brazil [7, 15, 25] and one in Jamaica [10]. Rates of affective psychosis in Brazil were similar in three independent samples and time points, ranging from 5.9 per 100,000 person-years (95%CI: 5.7–7.7) in a population-based study in Sao Paulo [7] to 7.3 (95%CI 7.2–7.4) in a nationwide study based on hospitalised admissions [25]. In Jamaica, Hickling and Rodgers-Johnson [10] reported a lower incidence of affective psychoses, derived from 15 cases with a crude rate of 1.1 per 100,000 person-years (95%CI 0.7–1.8).
Variance by continent
The Americas
Nine core citations estimated incidence rates in LMIC settings in the Americas, including four from Brazil (two national [25, 36], two catchment area-based [7, 15]), two national studies from Suriname [23, 27], two national studies in Costa Rica [26] and Jamaica [10], and a study in the state of Caldas, Colombia [38]. In Brazil, a nationwide study of all first psychiatric admissions between 1960 and 1974 reported the treated incidence of schizophrenia and affective psychoses over time for men and women separately [25]. Reported crude treated rates of both sets of disorders generally increased over the time period, with overall rates of 25.9 cases of schizophrenia per 100,000 person-years (95%CI 25.7–26.0), and 7.3 cases of affective psychoses (95%CI 7.2–7.4). Incidence rates of schizophrenia were higher in this study than the overall rate of all clinically relevant psychotic disorders estimated from two catchment area studies in Sao Paulo (15.8 per 100,000 person-years; 95%CI 14.3–17.6) [7], and Ribeirao Preto (21.5; 95%CI 19.8–23.3) [15], which—in contrast to the nationwide study [25]—both employed standardised research-based diagnoses, population-based case finding approaches, a leakage design to ascertain potentially missed cases by the initial screen, and restricted the age range to 18–64 years old. A further nationwide study detailing psychiatric hospitalisations reported an age-sex standardised incidence rate of NAPD of 82.9 (95%CI 71.6–94.1) per 100,000 person-years, based on an assessment of clinical records only [36].
Two separate nationwide studies of the incidence of NAPDs in Suriname reported similar crude (16.1 [27] and 16.8 [23] per 100,000 person-years, respectively) and age-sex standardised rates (16.8 [27] and 17.7 [23], respectively) for people aged 15–54 years old, albeit using different designs. Hanoeman et al.’s earlier study [27] was based on all first admissions to the country’s only psychiatric hospital, whilst Selten et al.’s [23] study also identified cases through primary care. Hickling et al. [10] reported higher incidence rates of NAPD (23.6 per 100,000 person-years; 95%CI: 21.2–26.3) and schizophrenia (20.9; 95%CI 18.6–23.5) in a comparable nationwide study in Jamaica. An earlier nationwide study in Costa Rica reported high hospitalised rates of schizophrenia for people aged 15 years and older (48.2 per 100,000 person-years; 95%CI 46.5–50.0) based on administrative data using clinical diagnoses, with a similar estimate of crude incidence (45 per 100,000 person-years; 95%CI 42.4–47.8) found using a similar design between 2005 and 2018 in Colombia [38].
Asia
Five core citations estimated the incidence of NAPD in Asia, three based in India [8, 22, 39], and two in China [33, 35]. Data from the WHO 10-country study [8] (also [9, 29]) suggested that crude incidence rates of NAPD were similar in rural and urban Chandigarh (rural: 42.0 per 100,000 person-years; 95%CI 32.2–54.8; urban: 35.0; 95%CI 29.9–41.0), and perhaps slightly higher than in Moscow (former USSR; 28.0; 95%CI 24.4–32.2). These patterns were similar for the incidence of schizophrenia. Comparably high crude and age-sex standardised incidence rates of all psychotic disorders (crude [derived]: 43.0; 95%CI 33.6–54.9; standardised: 45.9; 95%CI 34.5–57.3) and schizophrenia (crude [derived]: 24.8: 18.0–34.3; standardised: 27.2; 95%CI 18.3–36.0) were reported in provisional data from the INTREPID-I study in Chengalpattu, India [39]. In China, one study from Haidian District (Beijing) reported a lower treated incidence of schizophrenia (11.2; 95%CI 8.0–15.6), ascertained between 1975 and 1981 (also see [34]), while a second study from Guangdong province reported a crude incidence of 24.9 (95%CI 23.6–26.1) with cases recruited between 1978 and 1987 [35].
Africa
Three core citations provided incidence data in Africa: two from Nigeria [28, 39], and a third in South Africa [24]. In Nigeria, a 30-day study conducted in April 1980 reported all treated cases diagnosed with schizophrenia for the first time in the only psychiatric hospital in the state of Anambra [28]. We estimated the derived incidence as 14.0 new cases per 100,000 person-years (95%CI 11.0–17.7; see Supplemental Materials, Sect. 7). A more recent study [39] in Ibadan, Nigeria, used a population-based case finding approach to estimate the crude (derived) and age-sex standardised incidence of schizophrenia as 31.8 per 100,000 person-years (95%CI 23.6–42.9) and 27.5 (95%CI 19.1–36.0), respectively. Corresponding rates for all clinically relevant psychotic disorders were 35.5 (95%CI 26.7–47.1) and 31.2 (95%CI 22.2–40.3), respectively [39]. This was similar to the crude treated incidence reported in the District of uMgungundlovu, KwaZulu-Natal, South Africa (31.5; 95%CI 27.0–36.8) [24].
Europe
We identified five citations which provided estimates of incidence rates in the former USSR (all Moscow [8, 9, 30,31,32]), including three core citations [8, 31, 32]. The earliest report estimated the overall treated incidence of ICD-8 schizophrenia in people aged 15–44 years as 19.1 per 100,000 person-years (no SE provided) [32], as one of the field centres participating in the WHO International Pilot Study of Schizophrenia. A lower incidence in the population aged 15–54 was reported in the WHO 10-country study as 12.0 (95%CI 9.7–14.9) [8], with a higher rate of NAPDs in that sample (28.0; 95%CI 24.4–32.2). Finally, in 1982, Rotshtein [31] reported the incidence of paranoid schizophrenia as 1.7 per 100,000 person-years (no SE provided) [31].
Variance by sex
Five core citations reported incidence rates for men and women separately [8, 10, 15, 25, 32]. Point estimates of incidence for all psychotic disorders [15], and schizophrenia [10, 25, 32] were generally higher for men than women, except in the WHO 10-country study [8], which found no consistent evidence of this effect [8] (Supplemental Materials, Sect. 8). In Brazilian national data, there was evidence of higher rates of affective psychoses in women than men across a 14-year period [25].
Quality appraisal
Study quality ranged from one to six (out of seven; Table 1, Supplemental Table 2), with a median of four (IQR: 3–6) from 16 of the 18 core citations for which a full-text review could be performed (Supplemental Materials, Sect. 6). We found no evidence of correlation (ρ = 0.32; p = 0.23) between reported study quality and median year of case ascertainment (Supplemental Fig. 1). We found no evidence of substantive correlation between incidence rates of schizophrenia and study quality (ρ = − 0.29; p = 0.34) or time period (ρ = 0.41; p = 0.16). We could not perform formal meta-regression on these data due to the small number of available data points.
Small study effects
Funnel plots for NAPD, schizophrenia and all FEP (Supplemental Figs. 2–4) did not provide evidence of small study effects for patterns of incidence on these outcomes. We only had sufficient citations to formally test small study effects via an Egger’s test for schizophrenia (N = 13; p = 0.70), which suggested no evidence of funnel plot asymmetry. There may, however, have been some evidence that smaller studies of lower rates of all clinically relevant psychotic disorders were absent from the published literature (Supplemental Fig. 4).
Discussion
Summary of main results
Our systematic review identified 23 citations published over a 60-year period on the incidence of NAPD in general adult population studies conducted in LMICs. This yield was limited to 19 settings from just ten countries, highlighting the dearth of evidence on the incidence and distribution of NAPD in LMICs. This paucity of evidence was further compounded by a lack of nationwide samples (only five settings) limiting knowledge to a small number of regions. Three-quarters of the identified studies were based on data collected with a mid-point of case ascertainment before 2003, and 50% before 1990. Studies were also characterised by high heterogeneity in research methods, diagnostic procedures and outcomes, and study quality.
Strengths and limitations of our review
Strengths of our review included an inclusive search strategy, based on previously-validated methods [2, 4] which followed the principles of the Cochrane Library, PRISMA guidelines, and prospective registration of our protocol on PROSPERO (Supplemental Materials, Sect. 9).
Limitations of our review include omission of data from one citation which may have met our inclusion based on its title (no abstract available) [40], despite exhaustive searches. Whilst we specified no exclusion criteria based on language, we limited our search to English language databases, and cannot exclude omission of relevant studies not indexed by these repositories. Despite this, we found no strong evidence of small study effects. We grouped studies based on broad diagnostic criteria, which masked variability in exact outcomes studied and diagnostic classification systems used in individual citations (Table 1). Although our primary diagnostic outcome was NAPD, we do not believe we will have omitted many studies of other clinically relevant non-organic psychotic disorders, since our search terms included “schizophrenia”, “psychosis”, “mental illness/disorder” and related variants (Supplemental Materials, Sect. 1). The study quality tool we used may have not captured total study quality; for example, it did not allow us to rate citations which failed to report the numerator (case) sample sizes for reported rates [8,9,10, 29,30,31,32]. The Cochrane filter on LMICs is based on current categorisations, making it possible we omitted studies conducted in countries which were subsequently redefined as HICs. Finally, we might have missed studies that do not explicitly state incidence rates, but which might contain sufficient information to derive them. However, at each stage of the selection process we aimed to be inclusive and only excluded true negatives. At full-text stage this included ascertaining if citations included sufficient data to derive incidence rates.
Meaning of the findings in context
To the best of our knowledge, this is the first standalone systematic review of the incidence of psychotic disorders in LMICs. Previous international reviews have not separately synthesised results from LMIC settings [3, 4], and either covered a narrow time period [4] or were restricted to schizophrenia [3]. The four- to fivefold variation in rates of NAPD and schizophrenia observed in LMICs is consistent with variance reported in earlier global reviews [3, 4], and with a synthesis of the available evidence in a multi-country study [29].
We observed heterogeneous methods and incidence estimates in LMICs, similarly to Morgan et al. [39], though with a more up-to-date summary of the available evidence. There was limited evidence that study quality had risen over time, although study quality was not correlated with variation in reported rates. Nevertheless, the comparison of incidence rates of psychotic disorders is predicated on sufficient commonality across methods to permit valid inferences about the distribution of psychotic disorders by person and place. While some basic tenets were evident across most studies identified here (defined catchment areas, accurate denominator, reporting of inclusion criteria), other methodological features were more variably applied (Supplemental Table 2).
Some of these issues may have particularly profound effects on estimating and comparing incidence rates in LMICs. For example, studies reliant on detecting cases solely presenting to psychiatric care [10, 25, 26, 28, 33] may underestimate the true population incidence of psychotic disorders, particularly in countries where formal mental health resources are more limited, or where help-seeking takes place in traditional or informal settings. In our review, evidence on this issue was limited. Two nationwide studies in Suriname reported similar rates of NAPD a decade apart [23, 27], whether case finding was restricted to secondary psychiatric care or extended to include primary care [23]. In contrast, two studies in Nigeria estimated a twofold difference in schizophrenia incidence depending on whether case ascertainment was restricted to first contact with psychiatric care [28] or used a population-based case finding approach [39]. This [39, 41], and other incidence initiatives underway in South Africa [42] and Iran [43], suggest that epidemiological methods can be adapted in different contexts to enable reliable case ascertainment to accurately estimate incidence in different contexts.
Methodological heterogeneity in incidence studies of psychotic disorders is by no means limited to LMICs [4], but has arguably become an endemic issue [15, 44], inhibiting progress in the field. Even where studies adopt common methodological procedures, our review highlights how their variable operationalisation may hinder meaningful comparisons. For example, although most studies defined their age ranges, this was variably applied (i.e. 15–44 [32], 15–49 [24], 15–54 [8,9,10, 23, 27, 29], 18–64 [7, 15, 39], 18–90 [38], the entire adult population [22, 26, 33], or entire population [25]). Finally, comparisons in this review were largely limited to crude rates, with only four core citations [15, 23, 27, 39] (26.7%) reporting age-sex standardised rates, an issue not limited to LMICs. Universal reporting of standardised rates will facilitate better quantification and exploration of heterogeneity in incidence across different settings.
To make meaningful progress in this field of psychosis epidemiology, we recommend two priorities. First, more investment is made to estimate the true incidence of all non-organic psychotic disorders worldwide, expanding beyond the relatively narrow set of countries where research has been conducted to date. Whereas around 87% of the global population lives in LMICs, only 2.4% of mental health research funding is currently spent on examining mental health in these settings [45]. Second, we recommend the development and adoption of a common epidemiological framework and guidelines for epidemiological studies to estimate the incidence of psychotic disorders reliably and comparably across diverse contexts. These priorities should be aligned, by funding ambitious, global consortia to establish a network of researchers collaborating to simultaneously estimate the incidence of psychosis in different settings, providing a foundation for further investigation of the aetiological determinants of, and inequalities in, variability in risk, course, and outcomes following the onset of psychosis across the globe. These efforts would aid and inform other global initiatives, including the World Health Survey and Global Burden of Diseases, which respectively, provide new understanding about the prevalence of subclinical psychotic symptoms [12, 13] and model-based projections of the likely incidence of disorder in different countries [46]. All such approaches are imperfect, but triangulating their findings would overcome limitations of current epistemological inferences about the distribution and determinants of the incidence of psychotic disorders, predominantly based on evidence generated in HICs.
Data Availability
The dataset generated and analysed in this study is publicly available via the Open Science Framework.Link to the project page: https://osf.io/ahb3q/
References
McGrath JJ (2007) The surprisingly rich contours of schizophrenia epidemiology. Arch Gen Psychiatry 64:14–16. https://doi.org/10.1001/archpsyc.64.1.14
Kirkbride JB, Errazuriz A, Croudace TJ et al (2012) Incidence of schizophrenia and other psychoses in England, 1950–2009: a systematic review and meta-analyses. PLoS ONE 7:e31660. https://doi.org/10.1371/journal.pone.0031660
McGrath J, Saha S, Welham J et al (2004) A systematic review of the incidence of schizophrenia: the distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC Med 2:1–22. https://doi.org/10.1186/1741-7015-2-13
Jongsma HE, Turner C, Kirkbride JB, Jones PB (2019) International incidence of psychotic disorders, 2002–17: a systematic review and meta-analysis. Lancet Public Health 4:e229–e244. https://doi.org/10.1016/S2468-2667(19)30056-8
Selten JP, Van Der Ven E, Termorshuizen F (2020) Migration and psychosis: a meta-analysis of incidence studies. Psychol Med 50:303–313. https://doi.org/10.1017/S0033291719000035
March D, Hatch SL, Morgan C et al (2008) Psychosis and place. Epidemiol Rev 30:84–100. https://doi.org/10.1093/epirev/mxn006
Menezes PR, Scazufca M, Busatto G et al (2007) Incidence of first-contact psychosis in São Paulo, Brazil. Br J Psychiatry 191:2–7. https://doi.org/10.1192/bjp.191.51.s102
Jablensky A, Sartorius N, Ernberg G, et al (1992) Schizophrenia: manifestations, incidence and course in different cultures A World Health Organization Ten-Country Study. England
Sartorius N, Jablensky A, Korten A et al (1986) Early manifestations and first-contact incidence of schizophrenia in different cultures: a preliminary report on the initial evaluation phase of the WHO Collaborative Study on Determinants of Outcome of Severe Mental Disorders. Psychol Med 16:909–928. https://doi.org/10.1017/S0033291700011910
Hickling FW, Rodgers-Johnson P (1995) The incidence of first contact schizophrenia in Jamaica. Br J Psychiatry 167:193–196. https://doi.org/10.1192/bjp.167.2.193
Mortensen PB, Pedersen CB, Westergaard T et al (1999) Effects of family history and place and season of birth on the risk of schizophrenia. N Engl J Med 340:603–608
DeVylder JE, Kelleher I, Lalane M et al (2018) Association of urbanicity with psychosis in low- and middle-income countries. JAMA Psychiat 75:678–686. https://doi.org/10.1001/jamapsychiatry.2018.0577
Kirkbride JB, Keyes KM, Susser E (2018) City living and psychotic disorders—implications of global heterogeneity for theory development. JAMA Psychiat. https://doi.org/10.1001/jamapsychiatry.2018.2640
González-Valderrama A, Jongsma HE, Mena C et al (2020) The incidence of non-affective psychotic disorders in Chile between 2005 and 2018: results from a national register of over 30 000 cases. Psychol Med. https://doi.org/10.1017/S0033291720002664
Jongsma HE, Gayer-Anderson C, Lasalvia A et al (2018) Treated incidence of psychotic disorders in the multinational EU-GEI study. JAMA Psychiat 75:36–46. https://doi.org/10.1001/jamapsychiatry.2017.3554
Cochrane Training LMIC Filters | Cochrane Effective Practice and Organisation of Care. https://epoc.cochrane.org/lmic-filters. Accessed 14 Dec 2020
The World Bank World Bank Country and Lending Groups. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed 15 Dec 2020
Institute for Health Metrics and Evaluation GBD 2019 Data. In: Global Health Data Exchange. https://ghdx.healthdata.org/. Accessed 15 Aug 2022
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Cochrane Training Chapter 10: Analysing data and undertaking meta-analyses. https://training.cochrane.org/handbook/current/chapter-10. Accessed 15 Dec 2020
R Development Core Team (2013) R: a language and environment for statistical computing
Rajkumar S, Padmavathi R, Thara R, Menon MS (1993) Incidence of schizophrenia in an urban community in Madras. Indian J Psychiatry 35:18–21
Selten J-P, Zeyl C, Dwark Asing R et al (2005) First-contact incidence of schizophrenia in Surinam. Br J Psychiatry 186:74–75. https://doi.org/10.1192/bjp.186.1.74
Burns JK, Esterhuizen T (2008) Poverty, inequality and the treated incidence of first-episode psychosis. An ecological study from South Africa. Soc Psychiatry Psychiatr Epidemiol 43:331–335. https://doi.org/10.1007/s00127-008-0308-2
Caetano R (1981) First admission to psychiatric facilities in Brazil, 1960–1974. Bull Pan Am Health Organ 15:148–159
Handal NN, Dodds JH (1997) Rates of first hospital admissions for schizophrenia in Costa Rica. Revista Panamericana de Salud Publica/Pan American Journal of Public Health 1:426–434
Hanoeman M, Selten J-P, Kahn RS (2002) Incidence of schizophrenia in Surinam. Schizophr Res 54:219–221. https://doi.org/10.1016/s0920-9964(01)00269-9
Ihezue UH (1982) Some observations and comments on the psychosocial profile of first-ever referrals to the psychiatric hospital, Enugu, Nigeria. Acta Psychiatr Scand 65:355–364. https://doi.org/10.1111/j.1600-0447.1982.tb00856.x
Wig NN, Varma VK, Mattoo SK et al (1993) An incidence study of schizophrenia in India. Indian J Psychiatry 35:11–17
Tsirkin SI (1987) International study of schizophrenia based on a WHO program. Incidence of schizophrenia. Zh Nevropatol Psikhiatr Im S S Korsakova 87:1203–1207
Rotshtein VG (1982) Incidence of paranoid schizophrenia. Zh Nevropatol Psikhiatr Im S S Korsakova 82:91–98
Liberman II (1974) The incidence of schizophrenia (materials from a clinico-epidemiologic study). Zh Nevropatol Psikhiatr Im S S Korsakova 74:1224–1233
Chen CH, Yucun S, Xi T et al (1984) Incidence and prevalence of schizophrenia in a community mental health service from 1975 to 1981. Chin J Neurol Psychiatry 17:321–324
Shen Y, Zhang W, Shu L et al (1987) A survey of mental disorders in a suburb of Beijing. Int J Ment Health 16:75–80. https://doi.org/10.1080/00207411.1987.11449071
Huang L-S, Chen Y-F, Lin X-Z, Wu Y-T (1990) A 10-year investigation on incidence of schizophrenia in community. Chin J Nerv Ment Dis 16:100–103
da Rocha HA, Reis IA, da Cunha Santos MA, et al (2021) Psychiatric hospitalizations by the Unified Health System in Brazil between 2000 and 2014. Rivesta de Saude Publica 55
Del-Ben CM, Shuhama R, Loureiro CM et al (2019) Urbanicity and risk of first-episode psychosis: incidence study in Brazil. Br J Psychiatry 215:726–729. https://doi.org/10.1192/bjp.2019.110
Song J, Ramírez MC, Okano J et al (2022) Geospatial analysis reveals distinct hotspots of severe mental illness. medRxiv. https://doi.org/10.1101/2022.03.23.22272776
Morgan C, John S, Esan O et al (2016) The incidence of psychoses in diverse settings, INTREPID (2): a feasibility study in India, Nigeria, and Trinidad. Psychol Med 46:1923–1933. https://doi.org/10.1017/S0033291716000441
Munjiza M (1987) An estimation of schizoaffective disorders incidence in the group of schizophrenic psychoses. Medicinski Anali 13:59–65
Roberts T, Morgan C, Gureje O et al (2020) T241. Incidence and socio-demographic/clinical characteristics of psychotic disorders in India, Nigeria and Trinidad: preliminary baseline findings from intrepid II. Schizophr Bull 46:S324–S324. https://doi.org/10.1093/SCHBUL/SBAA029.801
Veling W, Burns JK, Makhathini EM et al (2019) Identification of patients with recent-onset psychosis in KwaZulu Natal, South Africa: a pilot study with traditional health practitioners and diagnostic instruments. Soc Psychiatry Psychiatr Epidemiol 54:303–312. https://doi.org/10.1007/s00127-018-1623-x
Farhang S, Ghaemmaghami M, Noorazar G et al (2020) T152. Azeri recent onset acute phase psychosis survey (Aras Cohort): preliminary reports from an observational cohort of first episode psychosis in Iran. Schizophr Bull 46:S288–S289. https://doi.org/10.1093/schbul/sbaa029.712
Hogerzeil SJ, van Hemert AM, Veling W, Hoek HW (2016) Incidence of schizophrenia among migrants in the Netherlands: a direct comparison of first contact longitudinal register approaches. Soc Psychiatry Psychiatr Epidemiol 52:147–154. https://doi.org/10.1007/s00127-016-1310-8
Woelbert E, Lundell-Smith K, Grant J, Kemmer D (2020) The inequities of mental health research (IAMHRF). Montreal
Hay SI, Abajobir AA, Abate KH et al (2017) Global, regional, and national disability-adjusted life-years (DALYs) for 328 diseases and injuries and healthy life expectancy (HALE) for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. The Lancet 390:1260–1344. https://doi.org/10.1016/S0140-6736(17)32130-X
Acknowledgements
We are grateful to Deborah Marletta, librarian at UCL, for assisting with the development of the search terms applied to the databases included in this review.
Funding
Funding for this project was obtained as part of the Delineating the Epidemiology of first episode Psychosis In Chile: a nationwide register study of 30,000 incidenT cases between 2005 and 2018 (the DEPICt study), under the UCL ‘Grand Challenges’ small grants scheme (2019). RJ-BB, JBK and HEJ are supported by the National Institute for Health Research University College London Hospital Biomedical Research Centre. LV acknowledges financial support from the National Institute for Health Research (NIHR) Biomedical Research Centre for Mental Health at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Author information
Authors and Affiliations
Contributions
RJ-BB carried out searches, extracted, managed and interpreted data and contributed to writing the manuscript. AG-V carried out quality assessment and provided feedback on the manuscript. TD obtained funding, carried out analyses and provided feedback on the manuscript. LV carried out quality assessment and provided feedback on the manuscript. JBK conceived of the research idea, obtained funding and wrote the manuscript. HEJ managed and interpreted data, mediated in quality assessment and wrote the manuscript. RJ-BB and HEJ verified underlying data, all study authors had full access to the data and accept responsibility to submit for publication. The curated spreadsheet for this systematic review containing all meta-data and rate-level data is available on our pre-print server under a Creative Commons CC-BY open access arrangement (URL: 100.17605/OSF.IO/AHB3Q). The pre-registered protocol for this review is available on the PROSPERO website: CRD42020179678.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare they have no conflicts of interest to report.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bastien, R.JB., Ding, T., Gonzalez-Valderrama, A. et al. The incidence of non-affective psychotic disorders in low and middle-income countries: a systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol 58, 523–536 (2023). https://doi.org/10.1007/s00127-022-02397-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00127-022-02397-6