Abstract
Aims/hypothesis
Continuous glucose monitoring (CGM) provides comprehensive information on the exposure to dysglycaemia. This study aimed to investigate the threshold of hyperglycaemia related to mortality risk in critically ill patients using CGM technology.
Methods
A total of 293 adult critically ill patients admitted to intensive care units of five medical centres were prospectively included between May 2020 and November 2021. Participants wore intermittently scanned CGM for a median of 12.0 days. The relationships between different predefined time above ranges (TARs), with the thresholds of hyperglycaemia ranging from 7.8 to 13.9 mmol/l (140–250 mg/dl), and in-hospital mortality risk were assessed by multivariate Cox proportional regression analysis. Time in ranges (TIRs) of 3.9 mmol/l (70 mg/dl) to the predefined hyperglycaemic thresholds were also assessed.
Results
Overall, 66 (22.5%) in-hospital deaths were identified. Only TARs with a threshold of 10.5 mmol/l (190 mg/dl) or above were significantly associated with the risk of in-hospital mortality, after adjustment for covariates. Furthermore, as the thresholds for TAR increased from 10.5 mmol/l to 13.9 mmol/l (190 mg/dl to 250 mg/dl), the hazards of in-hospital mortality increased incrementally with every 10% increase in TARs. Similar results were observed concerning the associations between TIRs with various upper thresholds and in-hospital mortality risk. For per absolute 10% decrease in TIR 3.9–10.5 mmol/l (70–190 mg/dl), the risk of in-hospital mortality was increased by 12.1% (HR 1.121 [95% CI 1.003, 1.253]).
Conclusions/interpretation
A glucose level exceeding 10.5 mmol/l (190 mg/dl) was significantly associated with higher risk of in-hospital mortality in critically ill patients.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
In critically ill patients, hyperglycaemia is common and is associated with poor outcomes [1, 2]. However, the results of the landmark Leuven study [3], which ushered in ‘tight glycaemic control’, were not reproduced in subsequent multicentre RCTs and meta-analyses [4,5,6,7,8]. Consequently, there is currently no firm consensus about how to manage blood glucose levels in critically ill patients.
Understanding the relationship between the glucose spectrum and clinical outcomes is fundamental for setting the glucose target. Compared with traditional blood glucose monitoring measurements, continuous glucose monitoring (CGM) provides a more complete glycaemic profile. Furthermore, the use of CGM in hospitals has been accelerated due to the COVID-19 pandemic [9,10,11]. Although the available evidence is limited, CGM has the potential to reduce the burden of nurses and may reduce hypoglycaemia incidence in critically ill patients [12,13,14,15]. Therefore, CGM provides new opportunities for more comprehensive and informative investigation of the association between hyperglycaemia and outcomes in critically ill patients.
Moreover, establishment of the core CGM metric is important for successful utilisation of CGM technology in routine clinical practice among critically ill patients. In recent years, the time in range (TIR), which refers to the percentage of time spent in an acceptable glucose range, has been popularised as an important measure for interpreting glucose data provided by CGM devices, with TIRs having been agreed upon by international guideline/consensus for use of diabetes management [16,17,18]. More recently, a consensus statement for CGM metrics in inpatient studies recommended TIRs as core outcome measures in the hospital setting [19]. With respect to the critically ill population, previous studies have observed a significant inverse association between time in range (TIR) and mortality risk, based on intermittent capillary, venous or arterial blood sampling data [20,21,22]. However, there is a lack of evidence linking TIR measured by CGM data to poor prognosis in critically ill patients. More importantly, the glucose range used to define TIR in this population remains to be explored [23, 24]. Therefore, based on CGM data, the current study examined the relationships between different predefined time above ranges (TARs)/TIRs using various glucose thresholds and the risk of in-hospital mortality in critically ill patients.
Methods
Study design and population
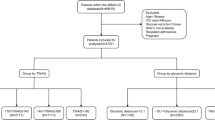
The INDIGO-ICU (INDices of contInuous Glucose monitoring and adverse Outcomes in Intensive Care Units) study is a multicentre, prospective and observational cohort study conducted in mixed medical/surgical intensive care units (ICUs) of five medical centres in Shanghai. It was designed to longitudinally examine the effects of quality of glucose control assessed by CGM on mortality risk in critically ill patients. The complete study protocol was approved by the Research Ethics Committees of Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, and was in accordance with the Helsinki Declaration principles. Written informed consent was obtained from all participants.
Patients were consecutively recruited between May 2020 and November 2021. The following inclusion criteria were applied: (1) age ≥18 years; and (2) expected to stay in the ICU for at least 3 days. Exclusion criteria were as follows: (1) readmission to the ICU; (2) CGM data for fewer than 24 h; (3) receiving paracetamol (acetaminophen) >4 g/day or receiving high-dose ascorbic acid [10]; and (4) an admission diagnosis of hyperosmolar hyperglycaemic state or diabetic ketoacidosis. All participants, of Asian ethnicity, were drawn from various rural and urban regions throughout China (mostly from Shanghai). A total of 293 participants were included in the final analysis.
Glucose control strategy
A standard ICU glucose control protocol was adopted. In accordance with ADA guidelines [16], the blood glucose target was 7.8–10.0 mmol/l (140–180 mg/dl) in our study. Blood glucose testing was performed using venous or capillary blood. The frequency of blood glucose monitoring ranged from hourly to every 4–6 h based on clinical need. Continuous i.v. administration of short-acting insulin (Tianmailin; Heifei Tianmai Biotechnology Development Co., China) using a micropump with a 1 U/ml concentration was initiated when blood glucose exceeded 10 mmol/l (180 mg/dl) for two successive readings. The initial insulin rates were recommended to be set using a sliding scale method, ranging from 2 to 6 U/h, according to the measured blood glucose levels (electronic supplementary material [ESM] Table 1). To avoid hypoglycaemia, we discontinued the i.v. infusion of insulin when blood glucose dropped below 7.8 mmol/l (140 mg/dl), and recommended i.v. administration of concentrated dextrose when blood glucose levels were below 3.9 mmol/l (70 mg/dl). For a single elevated blood glucose measurement, insulin was administered by s.c. injection (ESM Table 1).
Because CGM was not formally approved for hospital use, insulin adjustments in our study were guided via conventional blood glucose testing, and CGM data was blinded to the clinicians and nurses during the study period.
CGM
We used FreeStyle Libre Pro Flash CGM (Abbott Diabetes Care, Alameda, CA, USA), a factory-calibrated sensor, for blinded s.c. interstitial glucose monitoring. The sensors were inserted on the first day of ICU admission, and then interstitial glucose levels were continuously measured every 15 min, generating a daily record of 96 glucose values for up to 14 days. TARs were calculated as the percentage of time above the glucose thresholds of 7.8, 8.3, 8.9, 9.4, 10.0, 10.5, 11.1, 11.7, 12.2, 12.8, 13.3 and 13.9 mmol/l (140, 150, 160, 170, 180, 190, 200, 210, 220, 230, 240 and 250 mg/dl) during the whole glucose monitoring period, denoted as TAR>7.8 to TAR>13.9. In addition, TIRs were calculated as the percentage of time in the glucose range between 3.9 mmol/l (70 mg/dl) and the same upper thresholds mentioned above during the same period, denoted as TIR3.9–7.8 to TIR3.9–13.9. Hypoglycaemia metrics including time below range (TBR) <3.9 mmol/l (70 mg/dl), TBR <3.0 mmol/l (54 mg/dl), AUC per day <3.9 mmol/l (70 mg/dl) and AUC per day <3.0 mmol/l (54 mg/dl) were calculated. Glycaemic variability metrics, including SD, CV and mean amplitude of glycaemic excursion (MAGE), were also calculated.
Data extraction and outcome
The primary outcome was in-hospital mortality, defined as the occurrence of death during the hospital stay consecutive to the first ICU admission and prior to discharge. The following clinically relevant data was extracted from the hospital electronic medical record system and the ICU’s comprehensive database: demographic information, anthropometric measures and laboratory results. The sex of the study participants was determined through self-report during the initial demographic data collection. Disease severity was assessed by using the extracted Acute Physiology Score of the Acute Physiology and Chronic Health Evaluation II (APACHE II) [25].
Statistical analysis
R version 4.0.3 (https://www.r-project.org) was used for the statistical analysis. Normality was tested by the Shapiro–Wilk Normality test first. Continuous variables were presented as mean ± SD or median (IQR, 25–75%) and categorical variables were presented as n (%). To compare the general characteristics of participants with or without outcome, t tests or Mann–Whitney U tests were conducted for normally or non-normally distributed continuous variables and χ2 tests were used for categorical variables. To investigate the upper threshold of glucose range, Cox proportional hazards regression was performed to assess the relationships of TAR>7.8 to TAR>13.9, with the risk of in-hospital mortality. Specifically, the values of TARs can be 0–100% and the HRs (95% CIs) were estimated by including different predefined TARs in the models separately as a continuous variable (per absolute 10% increase in TARs). In addition, the relationships of TIR3.9–7.8 to TIR3.9–13.9 with the risk of in-hospital mortality were also assessed, to further validate the threshold. Statistically significant covariates with a level of p<0.10, identified by univariate analysis and clinically relevant covariates based on prior literature (significant or not), were entered into the multivariate Cox regression model. The final model included age, sex, APACHE II score, the presence of diabetes, use of glucocorticoid in hospital and use of insulin in hospital. Then restricted cubic spline nested in the multivariate-adjusted Cox regression model was used to assess the dose–response relationship between levels of CGM metrics and in-hospital mortality. Moreover, explorative subgroup analyses in participants with and without pre-existing diabetes were performed. Two-tailed p values <0.05 were considered to indicate statistical significance.
Results
A total of 293 critically ill patients (198 male, 95 female) were included in the final analysis. The clinical characteristics of the study population are presented in Table 1. Briefly, the mean ± SD age of the participants was 68±15 years and the mean ± SD APACHE II score was 19±6. Among them, 69 (23.5%) participants had pre-existing diabetes. During follow-up, 66 in-hospital deaths were identified (22.5%). Of those, there were 49 (21.9%) in-hospital deaths among participants without diabetes and 17 (24.6%) among those with diabetes (p=0.75). Participants who died before hospital discharge showed significantly higher APACHE II score and were less likely to receive glucocorticoid in hospital (both p<0.05), compared with those who survived to discharge.
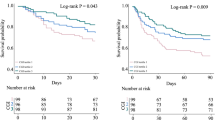
Overall, participants wore the sensor for a median (IQR) period of 12.0 (7.0–14.0) days. After adjusting for age, sex, APACHE II score, diabetes, use of glucocorticoid in hospital and use of insulin in hospital, only TARs with a glucose threshold of 10.5 mmol/l (190 mg/dl) or higher (TAR>10.5 to TAR>13.9) were significantly associated with in-hospital mortality risk (Table 2, Fig. 1). As the thresholds for TARs glucose ranges increased from 10.5 mmol/l to 13.9 mmol/l (190 mg/dl to 250 mg/dl), the HR for in-hospital mortality increased incrementally with every 10% increase in TARs (Table 2, Fig. 1). Similarly, only TIRs with upper thresholds from 10.5 mmol/l to 12.2 mmol/l (190 mg/dl to 220 mg/dl) (TIR3.9–10.5 to TIR3.9–13.9) were significantly and negatively associated with the risk of in-hospital mortality, with the hazards of in-hospital mortality increasing incrementally (ESM Table 2).
Forest plots illustrating HRs (95% CIs) for in-hospital mortality by each 10% increase in different CGM-derived TARs (%) defined using various glucose thresholds. *p<0.05, shown in red. The value of TARs can be 0–100% and the HRs (95% CIs) and p values were reported as per absolute 10% increment in TARs after adjustment for age, sex, APACHE II score, diabetes, use of glucocorticoid in hospital and use of insulin in hospital. The glucose thresholds shown on the x-axis are reported in units of both mmol/l and mg/dl (in parentheses)
Restricted cubic spline analysis suggested a significantly linear negative relationship between TIR3.9–10.5 and the risk of in-hospital mortality in critically ill patients (p for non-linear=0.193). Furthermore, restricted cubic spline curve showed that when TIR3.9–10.5 <52.7%, the risk of in-hospital mortality increases with decreasing TIR3.9–10.5 (Fig. 2). For per absolute 10% decrease in TIR3.9–10.5, the risk of in-hospital mortality was increased by 12.1% (HR 1.121 [95% CI 1.003, 1.253]) after adjustment for confounders (Fig. 2).
HRs (95% CIs) for in-hospital mortality by different levels of TIR (%) defined with a target range of 3.9–10.5 mmol/l (70–190 mg/dl) after adjustment for age, sex, APACHE II score, diabetes, use of glucocorticoid in hospital and use of insulin in hospital. The blue line represents the HR and the shaded blue area represents 95% CI. The dashed line indicates an HR of 1. Bars represent frequency (n)
Subgroup analyses stratified by pre-existing diabetes status showed that in participants without diabetes, only TARs with a threshold of 11.1 mmol/l (200 mg/dl) or above (except at 13.3 mmol/l [240 mg/dl]) were significantly associated with the risk of in-hospital mortality after adjustment for covariates, with HRs for in-hospital mortality increasing incrementally (Fig. 3a and ESM Table 3). In participants with diabetes, however, there were no statistically significant associations between different predefined TARs and mortality risk (Fig. 3b and ESM Table 4).
Forest plots illustrating HRs (95% CIs) for in-hospital mortality by different predefined TARs (%) among subpopulations stratified according to pre-existing diabetes status. (a) Participants without diabetes (n=224). (b) Participants with diabetes (n=69). *p<0.05, shown in red. The value of TARs can be 0–100% and the HRs (95% CIs) and p values were reported as per absolute 10% increment in TARs after adjustment for age, sex, APACHE II score, use of glucocorticoid in hospital and use of insulin in hospital. The glucose thresholds shown on the x-axis are reported in units of both mmol/l and mg/dl (in parentheses)
The relationships of CGM-derived hypoglycaemia metrics and glycaemic variability metrics with the risk of in-hospital mortality are presented in ESM Table 5. We found that higher SD (1-SD: HR 1.316 [95% CI 1.024, 1.692]) and MAGE (1-SD: HR 1.331 [95% CI 1.024, 1.730]) were significantly associated with higher risk of in-hospital mortality. However, the relationships of CV and hypoglycaemia metrics with the in-hospital mortality risk did not reach statistical significance (all p>0.05).
Discussion
In the current multicentre, prospective, observational study, we found that TARs with thresholds of 10.5 mmol/l (190 mg/dl) or higher were significantly related to the risk of in-hospital mortality in critically ill patients. The same threshold of hyperglycaemia associated with mortality risk was observed when considering TIRs with different upper thresholds. Therefore, the optimal upper glucose range for defining TIR in critically ill patients may be set near 10.5 mmol/l (190 mg/dl).
Understanding the relationship between the glucose spectrum and clinical outcomes is essential for setting the glucose target. The present results suggest that critically ill patients with sensor glucose level exceeding 10.5 mmol/l (190 mg/dl) may have an increased risk of mortality, similar to the findings of some previous RCTs targeting different blood glucose ranges [4,5,6]. For example, in the landmark NICE-SUGAR trial [4], critically ill patients assigned to intensive glycaemic management (4.4–6.1 mmol/l [80–110 mg/dl]) derived no significant treatment advantage compared with patients with more moderate glycaemic targets (7.8–10 mmol/l [140–180 mg/dl]) and had slightly but significantly higher mortality rate. Although debated, most professional societies currently recommend more ‘moderate’ glycaemic management for critically ill adults and suggest a blood glucose value of 10 mmol/l (180 mg/dl) or greater to trigger the use of insulin therapy [1, 16]. More recently, the large multicentre TGC-fast trial [26] showed that tight glucose control, in the context of delaying parenteral feeding to beyond the first week in ICU and the use of a performant algorithm, did not affect mortality and may protect liver and kidney function in critically ill patients, thus rekindling the long-debated question of glycaemic control in the ICU setting. Regarding this issue, our results seem to support a ‘moderate’ glycaemic management. However, it should be pointed out that the question of whether or not targeting a sensor glucose level <10.5 mmol/l (190 mg/dl) would reduce mortality risk remains to be addressed in future RCTs.
CGM has the potential to improve glucose control in the hospital setting, although core CGM metrics specific to inpatient care remains to be established [27]. Of the numerous metrics generated from CGM, TIRs have been recommended by international consensus statements and guidelines as important measures of glucose control [16, 17, 19]. With respect to the critically ill population, based on retrospective analysis of intermittent capillary, venous or arterial blood sampling data, TIR has been found to be negatively associated with the risk of mortality [20,21,22]. In addition, in a post hoc analysis of the SPRINT study data, TIR was observed to clearly discriminate the quality of glucose control between the SPRINT and Pre-SPRINT cohorts, despite similar median glucose values [28]. Compared with these previous studies, the main strength of the current study lies in the use of CGM data for the calculation of TIRs, which can provide more comprehensive information on the glucose profile throughout the day. Our results further provide evidence for the association between TIR/TAR measured by CGM data and mortality risk in critically ill patients. Moreover, our results indicate that 10.5 mmol/l (190 mg/dl) may be the appropriate upper limit of the ‘target’ glucose range used to define TIRs in critically ill patients. Taken together, TIRs, as valuable clinical measures, are worthy of more attention when using CGM technology and in the design of future interventional trials in critically ill patients. Of note, the ‘target’ glucose range used to define TIRs remains to be explored in different subpopulations based on clinical or participant-related outcomes [23, 24, 29, 30].
Besides, our results show that as the thresholds increase, every absolute 10% increase in TARs is related to incrementally higher risk of mortality. This could be explained by the effect of more ‘severe’ hyperglycaemia on mortality [31]. Compared with TIR, TAR is a more suitable metric for investigating the effect of hyperglycaemia, as it focuses on the relative exposure to hyperglycaemia, whereas TIR quantifies the relative exposure to ‘euglycaemia’ and hence reflects the risk of both hyperglycaemia and hypoglycaemia.
The main strengths of the current study include a prospective, multicentre study design and the use of 14 day CGM. However, there are also some limitations. First, it should be noted that the CGM device used in this study (FreeStyle Libre Pro) could be less accurate in the hypoglycaemia range [32], although this also applies to most other types of currently available CGM [33,34,35]. Therefore, the lower threshold of TIR, as well as the relationships between CGM-derived hypoglycaemia metrics and outcomes in critically ill patients, remains to be investigated in future studies, as new CGM technology with better performance in hypoglycaemia becomes available. Second, although we prospectively determined diabetes status at the onset of ICU admission based on all available information, the diabetes status adjusted in the model may not have been completely accurate. The potential influence of undiagnosed diabetes on our findings cannot be excluded. Third, due to the limited sample size of the subgroups stratified by diabetes status, the results of the subgroup analysis should be interpreted with caution. Future studies are warranted to further investigate the relationships between diabetes status, CGM metrics and outcomes in critically ill patients. Fourth, because of the limited scope of the medical records used in our study, the nutrition supply data was not available in this study.
In conclusion, we found that TARs/TIRs with an upper threshold of 10.5 mmol/l (190 mg/dl) or higher were significantly associated with the risk of in-hospital mortality in critically ill patients. These results suggest that critically ill patients with sensor glucose levels of 10.5 mmol/l (190 mg/dl) or higher may have an increased risk of mortality. In addition, based on our series of studies [29, 30], it is essential to acknowledge that when using TIR in clinical practice, different glucose ranges should be defined according to the characteristics of different populations, as well as treatment purpose. Future RCTs are warranted to determine whether targeting a sensor glucose level <10.5 mmol/l (190 mg/dl) would reduce the risk of mortality in critically ill patients.
Abbreviations
- APACHE II:
-
Acute Physiology and Chronic Health Evaluation II
- CGM:
-
Continuous glucose monitoring
- ICU:
-
Intensive care unit
- MAGE:
-
Mean amplitude of glycaemic excursion
- TAR:
-
Time above range
- TBR:
-
Time below range
- TIR:
-
Time in range
References
Evans L, Rhodes A, Alhazzani W et al (2021) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med 47(11):1181–247. https://doi.org/10.1007/s00134-021-06506-y
Alhatemi G, Aldiwani H, Alhatemi R, Hussein M, Mahdai S, Seyoum B (2022) Glycemic control in the critically ill: less is more. Cleve Clin J Med 89(4):191–9. https://doi.org/10.3949/ccjm.89a.20171
van den Berghe G, Wouters P, Weekers F et al (2001) Intensive insulin therapy in critically ill patients. N Engl J Med 345(19):1359–67. https://doi.org/10.1056/NEJMoa011300
Finfer S, Chittock DR, Su SY et al (2009) Intensive versus conventional glucose control in critically ill patients. N Engl J Med 360(13):1283–97. https://doi.org/10.1056/NEJMoa0810625
Griesdale DE, de Souza RJ, van Dam RM et al (2009) Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ 180(8):821–7. https://doi.org/10.1503/cmaj.090206
Preiser JC, Devos P, Ruiz-Santana S et al (2009) A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med 35(10):1738–48. https://doi.org/10.1007/s00134-009-1585-2
Kansagara D, Fu R, Freeman M, Wolf F, Helfand M (2011) Intensive insulin therapy in hospitalized patients: a systematic review. Ann Intern Med 154(4):268–82. https://doi.org/10.7326/0003-4819-154-4-201102150-00008
Poole AP, Finnis ME, Anstey J et al (2022) The effect of a liberal approach to glucose control in critically ill patients with type 2 diabetes: a multicenter, parallel-group, open-label randomized clinical trial. Am J Respir Crit Care Med 206(7):874–82. https://doi.org/10.1164/rccm.202202-0329OC
Galindo RJ, Aleppo G, Klonoff DC et al (2020) Implementation of continuous glucose monitoring in the hospital: emergent considerations for remote glucose monitoring during the COVID-19 pandemic. J Diabetes Sci Technol 14(4):822–32. https://doi.org/10.1177/1932296820932903
Agarwal S, Mathew J, Davis GM et al (2021) Continuous glucose monitoring in the intensive care unit during the COVID-19 pandemic. Diabetes Care 44(3):847–9. https://doi.org/10.2337/dc20-2219
Longo RR, Elias H, Khan M, Seley JJ (2022) Use and accuracy of inpatient CGM during the COVID-19 pandemic: an observational study of general medicine and ICU patients. J Diabetes Sci Technol 16(5):1136–43. https://doi.org/10.1177/19322968211008446
Krinsley JS, Chase JG, Gunst J et al (2017) Continuous glucose monitoring in the ICU: clinical considerations and consensus. Crit Care 21(1):197. https://doi.org/10.1186/s13054-017-1784-0
Preiser JC, Lheureux O, Thooft A, Brimioulle S, Goldstein J, Vincent JL (2018) Near-continuous glucose monitoring makes glycemic control safer in ICU patients. Crit Care Med 46(8):1224–9. https://doi.org/10.1097/CCM.0000000000003157
Yao Y, Zhao YH, Zheng WH, Huang HB (2022) Subcutaneous continuous glucose monitoring in critically ill patients during insulin therapy: a meta-analysis. Am J Transl Res 14(7):4757–67
Guerrero-Arroyo L, Faulds E, Perez-Guzman MC, Davis GM, Dungan K, Pasquel FJ (2023) Continuous glucose monitoring in the intensive care unit. J Diabetes Sci Technol 17(3):667–78. https://doi.org/10.1177/19322968231169522
ElSayed NA, Aleppo G, Aroda VR et al (2023) 6. Glycemic targets: standards of care in diabetes-2023. Diabetes Care 46(Suppl 1):S97-s110. https://doi.org/10.2337/dc23-S006
Battelino T, Danne T, Bergenstal RM et al (2019) Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 42(8):1593–603. https://doi.org/10.2337/dci19-0028
Battelino T, Alexander CM, Amiel SA et al (2023) Continuous glucose monitoring and metrics for clinical trials: an international consensus statement. Lancet Diabetes Endocrinol 11(1):42–57. https://doi.org/10.1016/S2213-8587(22)00319-9
Spanakis EK, Cook CB, Kulasa K et al (2023) A consensus statement for continuous glucose monitoring metrics for inpatient clinical trials. J Diabetes Sci Technol 19322968231191104. https://doi.org/10.1177/19322968231191104
Krinsley JS, Preiser JC (2015) Time in blood glucose range 70 to 140 mg/dl >80% is strongly associated with increased survival in non-diabetic critically ill adults. Crit Care 19(1):179. https://doi.org/10.1186/s13054-015-0908-7
Lanspa MJ, Krinsley JS, Hersh AM et al (2019) Percentage of time in range 70 to 139 mg/dL is associated with reduced mortality among critically Ill patients receiving IV insulin infusion. Chest 156(5):878–86. https://doi.org/10.1016/j.chest.2019.05.016
Ammar MA, Ammar AA, Wee T, Deshpande R, Band M, Akhtar S (2022) Relationship between glucose time in range in diabetic and non-diabetic patients and mortality in critically Ill patients. J Intensive Care Med 37(12):1625–33. https://doi.org/10.1177/08850666221098383
Kong APS, Lim S, Yoo SH et al (2023) Asia-Pacific consensus recommendations for application of continuous glucose monitoring in diabetes management. Diabetes Res Clin Pract 201:110718. https://doi.org/10.1016/j.diabres.2023.110718
Wan J, Lu J, Li C, Ma X, Zhou J (2023) Research progress in the application of time in range: more than a percentage. Chin Med J (Engl) 136(5):522–7. https://doi.org/10.1097/CM9.0000000000002582
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13(10):818–29. https://doi.org/10.1097/00003246-198510000-00009
Gunst J, Debaveye Y, Güiza F et al (2023) Tight blood-glucose control without early parenteral nutrition in the ICU. N Engl J Med 389(13):1180–90. https://doi.org/10.1056/NEJMoa2304855
Tian T, Aaron RE, Yeung AM et al (2023) Use of continuous glucose monitors in the hospital: the diabetes technology society hospital meeting report 2023. J Diabetes Sci Technol 17(5):1392–418. https://doi.org/10.1177/19322968231186575
Chase JG, Pretty CG, Pfeifer L et al (2010) Organ failure and tight glycemic control in the SPRINT study. Crit Care 14(4):R154. https://doi.org/10.1186/cc9224
Lu J, Home PD, Zhou J (2020) Comparison of multiple cut points for time in range in relation to risk of abnormal carotid intima-media thickness and diabetic retinopathy. Diabetes Care 43(8):e99–e101. https://doi.org/10.2337/dc20-0561
Shen Y, Fan X, Zhang L et al (2021) Thresholds of glycemia and the outcomes of COVID-19 complicated with diabetes: a retrospective exploratory study using continuous glucose monitoring. Diabetes Care 44(4):976–82. https://doi.org/10.2337/dc20-1448
Group. DCaCTDEoDIaCESR (2016) Mortality in type 1 diabetes in the DCCT/EDIC versus the general population. Diabetes Care 39(8):1378–83. https://doi.org/10.2337/dc15-2399
Galindo RJ, Migdal AL, Davis GM et al (2020) Comparison of the FreeStyle Libre Pro flash continuous glucose monitoring (CGM) system and point-of-care capillary glucose testing in hospitalized patients with type 2 diabetes treated with basal-bolus insulin regimen. Diabetes Care 43(11):2730–5. https://doi.org/10.2337/dc19-2073
Davis GM, Spanakis EK, Migdal AL et al (2021) Accuracy of dexcom G6 continuous glucose monitoring in non-critically ill hospitalized patients with diabetes. Diabetes Care 44(7):1641–6. https://doi.org/10.2337/dc20-2856
Aberer F, Hajnsek M, Rumpler M et al (2017) Evaluation of subcutaneous glucose monitoring systems under routine environmental conditions in patients with type 1 diabetes. Diabetes Obes Metab 19(7):1051–5. https://doi.org/10.1111/dom.12907
Pleus S, Heinemann L, Freckmann G (2018) Blood glucose monitoring data should be reported in detail when studies about efficacy of continuous glucose monitoring systems are published. J Diabetes Sci Technol 12(5):1061–3. https://doi.org/10.1177/1932296817753629
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Acknowledgements
The authors thank all research staff, students and patients who participated in this study.
Data availability
Restrictions apply to the availability of data generated or analysed during this study to preserve patient confidentiality or because they were used under licence. Data are however available from the corresponding authors upon reasonable request.
Funding
This work was funded by the Program of Shanghai Academic/Technology Research Leader (22XD1402300), the Shanghai ‘Rising Stars of Medical Talent’ Youth Development Program–Outstanding Youth Medical Talents (SHWSRS(2021)_099) and the Shanghai Research Center for Endocrine and Metabolic Diseases (2022ZZ01002).
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
JZ, WH and YL designed the study. SL, KF, XH, FH, MS and YZ collected the data. YW cleaned the data. YW and JL performed statistical analysis and wrote the draft of the manuscript. JZ, WH and YL edited the manuscript and SL, KF, XH, FH, MS and YZ reviewed it critically for important intellectual content. All authors read and approved the final manuscript. JZ is the guarantor of this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Y., Li, S., Lu, J. et al. Threshold of hyperglycaemia associated with mortality in critically ill patients: a multicentre, prospective, observational study using continuous glucose monitoring. Diabetologia 67, 1295–1303 (2024). https://doi.org/10.1007/s00125-024-06136-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-024-06136-1