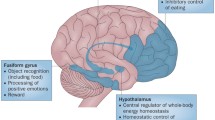
Abstract
Insulin exerts its actions not only on peripheral organs but is also transported into the brain where it performs distinct functions in various brain regions. This review highlights recent advancements in our understanding of insulin’s actions within the brain, with a specific emphasis on investigations in humans. It summarises current knowledge on the transport of insulin into the brain. Subsequently, it showcases robust evidence demonstrating the existence and physiological consequences of brain insulin action, while also introducing the presence of brain insulin resistance in humans. This pathophysiological condition goes along with an impaired acute modulation of peripheral metabolism in response to brain insulin action, particularly in the postprandial state. Furthermore, brain insulin resistance has been associated with long-term adiposity and an unfavourable adipose tissue distribution, thus implicating it in the pathogenesis of subgroups of obesity and (pre)diabetes that are characterised by distinct patterns of body fat distribution. Encouragingly, emerging evidence suggests that brain insulin resistance could represent a treatable entity, thereby opening up novel therapeutic avenues to improve systemic metabolism and enhance brain functions, including cognition. The review closes with an outlook towards prospective research directions aimed at further elucidating the clinical implications of brain insulin resistance. It emphasises the critical need to establish feasible diagnostic measures and effective therapeutic interventions.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Historical background: the discovery of brain insulin action and its contribution to systemic metabolism
The discovery of the brain’s role in whole-body metabolism goes back to the work of the French physiologist Claude Bernard in the mid-19th century. He discovered that puncturing the floor of the fourth ventricle in rabbits triggered glucosuria and resulted in the animals’ rapid demise, leading him to conclude that manipulating the brain can cause diabetes.
This foundational work was complemented by the discovery of insulin in 1921 and the subsequent discovery that it crosses the blood–brain barrier (BBB) in dogs [1]. The implications of this finding remained elusive, as insulin does not stimulate glucose uptake in neurons. Seminal works by Roth and colleagues in the mid-20th century demonstrated the presence of insulin receptors throughout the brain [2]. This finding prompted numerous experiments that revealed a complex interplay between the brain and systemic metabolism. The groundbreaking work of Woods and colleagues emphasised the pivotal role of brain insulin action, revealing that direct administration of insulin into the brain attenuated food intake and reduced body weight in a number of species, including baboons [3,4,5]. In line, brain-specific insulin-receptor knockout in rodents increased food intake and body weight, and induced systemic insulin resistance as well as hypertriglyceridaemia [6]. Another breakthrough was the discovery that brain insulin could modulate glucose metabolism via its influence on hepatic glucose production [7], presumably via the parasympathetic nervous system [8].
Subsequently, numerous studies characterised how brain insulin modulates whole-body glucose and lipid metabolism, and how it regulates appetite, energy expenditure and body weight. Through this scientific journey, brain insulin was discovered to impact multiple further brain functions such as cognition, memory and synaptic plasticity. This review mainly focuses on the role of brain insulin in human metabolism.
Transport of insulin into the human brain
The BBB is a selective, semi-permeable barrier around the microvasculature in the brain. It plays a crucial role in brain health by strictly controlling molecular passage between the bloodstream and the brain [9, 10]. The primary (but likely not the only) mechanism enabling insulin to enter the brain is receptor-mediated transcytosis [9,10,11] (i.e. binding to insulin receptors on the BBB and then moving across endothelial cells into the brain’s extracellular space [9, 10, 12]). In certain specialised brain regions, such as the arcuate nucleus of the hypothalamus, the BBB appears to be less dense [10]. Here, tanycytes, a cell type also expressing the insulin receptor [13], are required for insulin uptake [14].
Similar to animals [1], insulin is present in the cerebrospinal fluid (CSF) of humans [15]. This compartment is accessible for investigations of transport processes into the brain. Presence of insulin in the human CSF indicates that the hormone is transported from the bloodstream into the central nervous system. However, findings are still controversial as to whether tiny amounts of insulin may also be produced locally [16].
Insulin transport is not equally effective in all individuals. There appear to be a number of situations that either facilitate or hinder this transport, ultimately influencing insulin availability within the brain: insulin penetration into the CSF is lower in individuals with obesity [17, 18]. Furthermore, alterations in blood glucose levels acutely modulate the transport of peptide hormones, including insulin, into the CSF [18]. Thus, alterations in the transportation process across the BBB that requires the insulin receptor could account for the observed variations in insulin transport into the human CSF. In line, diminished transportation efficiency has been identified in individuals who present with systemic insulin resistance [19].
Ageing is also linked to a reduction in insulin transport into the CSF [20]. This decline may contribute to compromised brain insulin action, predisposing individuals to age-related cognitive dysfunction and neurodegenerative diseases [21]. This is supported by reduced CSF insulin concentrations in individuals with Alzheimer’s disease in some [22,23,24] but not all studies [24]. Yet, the precise underlying mechanisms governing the regulation of insulin transport into the human brain remain under investigation [11].
Evidence for brain insulin action and the existence of brain insulin resistance in humans
Evidence for brain insulin action
Just as in animals, insulin receptors are expressed in the human brain in neurons and other cell types (e.g. astrocytes) [12, 25, 26], teleologically arguing for a role of insulin in the brain.
Various techniques are used to stimulate brain insulin action in clinical research. The most physiological way is to measure the response to endogenous insulin that is released in response to food intake. However, numerous additional postprandial factors [27] hinder the dissection of insulin’s specific effects from other effects. A more selective approach is the i.v. infusion of insulin during hyperinsulinaemic–euglycaemic glucose clamps. However, this technique also cannot differentiate between peripheral and brain effects. One approach frequently used in clinical research to overcome this challenge is the administration of insulin by nasal spray [28]. This route delivers a substantial amount of insulin to the brain [29], while only small amounts enter the bloodstream [30, 31]. The quantity of insulin absorbed into the bloodstream is not sufficient to induce hypoglycaemia [30,31,32] and likely does not significantly contribute to the induced brain effects [31, 32]. Nevertheless, this insulin spillover must be taken into account when studying the potential impact of brain insulin on peripheral metabolism.
Modern neuroimaging techniques, such as functional MRI (fMRI), positron emission tomography (PET) and magnetoencephalography (MEG), have facilitated investigations into the effects of insulin on brain functions. MEG measures magnetic fields produced by the brain’s electrical activity. Early studies employing MEG demonstrated insulin’s impact on neuronal activity [33] and linked brain insulin effects to body weight [33, 34], metabolic factors [35] and genetic factors [36,37,38]. PET allows the assessment of metabolic processes, and most studies investigating insulin’s effects on the brain have employed the tracer fluorodeoxyglucose (FDG) to measure brain glucose uptake under insulin stimulation [39]. One study utilised the tracer raclopride to assess insulin’s effects on dopamine receptor availability [40].
Most studies on insulin action in the human brain have employed fMRI. In contrast to MEG, it provides higher spatial resolution for not only cortical but also subcortical regions. MRI enables detailed imaging of the brain’s anatomical structure and fMRI can also quantify functional aspects. With this technique, insulin-induced changes in regional brain activity and brain networks were detected [41]. Insulin-responsive networks and regions include areas critical for energy metabolism, eating behaviour, reward processes, mood and cognitive functions [25, 31, 41]. A recent systematic review of 58 RCTs using fMRI reported significant insulin effects in the inferior and middle frontal gyri, the dorsal striatum, the insula and the hypothalamus [31]. Further effects were reported in subcortical areas, including the hippocampus, in some but not all studies [31]. The insulin-responsive frontal gyri are part of the prefrontal cortex, which is involved in various high-level cognitive functions, including decision making and inhibitory control [31, 41, 42]. The dorsal striatum plays a crucial role in the brain’s reward system [40, 41, 43]. Its complex responses to insulin appear to contribute to the brain-derived modulation of peripheral insulin sensitivity [40, 44]. Notably, insulin modulates the tone of the principal neurotransmitter dopamine within this specific region of the human brain [40]. The insula is implicated in a wide range of functions [45] and plays a significant role in regulating the body’s homeostasis. Moreover, it is involved in the perception of bodily states, such as hunger and fullness [45], making it also essential for eating behaviour [31, 41]. The hypothalamus consists of various nuclei, some of which are critical for whole-body energy homeostasis, eating behaviour and body weight [46].
Hence, combining fMRI with nasal administration of insulin to assess insulin responses in regional cerebral blood flow in these areas could be a reliable and robust approach [31, 47] for quantifying brain insulin sensitivity in future trials.
Brain insulin resistance in humans
Using the techniques described above, it has become clear that insulin affects human brain activity. However, there is a substantial number of people with reduced or even absent brain response to insulin, a state termed ‘brain insulin resistance’ [25, 31, 48]. This condition is most commonly associated with overweight and obesity [25]. Additionally, further factors are also linked to brain insulin resistance, including normal ageing [41, 49, 50], circulating levels of NEFA [35] and different common genetic polymorphisms [36,37,38, 51, 52], most of which were discovered due to their association with body weight. Though, the direct role of these factors in causing brain insulin resistance is still under research.
Furthermore, recent neuroimaging data suggest sex differences in brain responses to insulin [47, 53]. In young women, insulin sensitivity of the hypothalamus appears to be rapidly modulated across the menstrual cycle with relative insulin resistance in the luteal phase [47]. However, not all studies on insulin action in the human brain report sex differences and the potential underlying mechanisms remain largely unexplored.
Further evidence for the effects of insulin in the human brain comes from functional studies, where nasal administration of insulin improved memory, altered eating behaviour and affected mood, at least in certain populations. These functions have been reviewed in greater detail elsewhere [28, 31]. In line with findings from neuroimaging studies, there are data suggesting sex differences in the effects of acute intranasal insulin delivery on eating behaviour and memory functions [28].
Effects of brain insulin action on peripheral metabolism
The first reports on genetic manipulation of brain insulin action in rodents suggested profound effects on peripheral metabolism [6]. Subsequent research in humans indicated that brain insulin action has a similar impact on the periphery, at least in individuals who are healthy and lean [25]. However, the precise mechanisms of signal transduction and regulation at the cellular level are still largely unexplored in humans.
A variety of clinical trials explored the metabolic effects of either nasal administration of insulin to the brain [44, 54,55,56,57,58] (for an overview see also electronic supplementary material [ESM] Tables 1, 2) or the pharmacological inhibition of brain insulin action [59, 60]. These studies indicated that brain insulin action has the potential to improve peripheral insulin sensitivity [44, 54, 55, 58, 59]. In most studies, this enhancement started approximately 45 min after nasal administration of insulin and was observed for at least 3 h [44, 55]. This outcome seems to involve several key mechanisms.
Brain insulin action suppresses endogenous glucose production [44, 56, 59], although the precise mechanisms and relative contribution is still under investigation (for review see, e.g. [61, 62]). In humans, this function seems only to occur under systemic hyperinsulinaemia but not at fasting insulin levels [63, 64]. Therefore, brain insulin might not directly inhibit hepatic glucose production, but rather enhance hepatic insulin sensitivity. This would facilitate the suppression of endogenous glucose production after meals when circulating insulin levels are high and insulin signalling in the brain occurs. In addition, brain insulin also acutely enhances liver energy metabolism and reduces liver fat content [64], presumably by promoting hepatic VLDL export (for review see, e.g. [65]). However, chronic intranasal insulin treatment did not change liver fat content but instead enhanced the liver’s secretion of branched-chain amino acids [57].
The impact of brain insulin action on human lipolysis is still not fully clear. While early studies suggested a suppressive effect [66], later trials that tightly controlled circulating insulin found no impact [64, 67, 68]. Considering the potent lipolysis-suppressing effect of even small increases in circulating insulin, it seems unlikely that brain-derived signals could substantially influence postprandial lipolysis under physiological circumstances with concurrently elevated insulin in both the circulation and the brain. Furthermore, brain insulin action appears to stimulate peripheral glucose uptake [44], thereby also contributing to improved peripheral insulin sensitivity (possible underlying mechanisms reviewed, e.g. in [61]).
Besides these effects on glucose handling, brain insulin acutely enhances glucose-stimulated insulin secretion from the pancreas [69]. Of note, this effect is exclusive for the second phase of insulin secretion and is closely linked to intact hypothalamic insulin sensitivity [69]. Mechanisms are likely similar to those underlying cephalic insulin responses [70, 71] and will therefore rely on the dense innervation of pancreatic islets [72]. While insulin resistance in the hypothalamus seems to hinder the acute stimulation of pancreatic insulin release, it was found to be paradoxically associated with insulin hypersecretion in response to oral glucose load in a cross-sectional study [73]. One possible explanation could be a long-term impairment of pancreatic inputs from the brain, as occurs in states of obesity and hypothalamic insulin resistance, which disrupts the balance of inputs to the beta cell. This imbalance could lead to an overabundance of stimulatory non-neuronal signals that promote insulin hypersecretion. Indeed, this has been observed in individuals with hypothalamic lesions [74] and may also occur in those with hypothalamic insulin resistance.
Possible impact of brain insulin resistance
Importantly, all these observations on the regulation of whole-body metabolism by brain insulin were made solely in lean individuals. These effects are diminished or absent in people who are overweight or obese or who have brain insulin resistance or type 2 diabetes (as reported for brain insulin effects on peripheral insulin sensitivity [44, 55], endogenous glucose production [44, 75], peripheral glucose uptake [44], liver energy metabolism [64], liver fat content [64] and pancreatic insulin secretion [69]). It is still unclear whether obesity itself or the often associated whole-body insulin resistance is responsible, as there are no studies on brain insulin action in individuals who are obese but still insulin sensitive. Longitudinal studies are needed to determine the sequence of development between peripheral and brain insulin resistance.
Potential (patho)physiological role of brain insulin in whole-body metabolism
Based on the above-described findings, we suggest a model for brain insulin’s role in peripheral glucose metabolism [25] (summarised in Fig. 1). Food intake triggers pancreatic insulin release, which crosses the BBB and reaches the brain. Here, it acts on specific neurons (e.g. in the hypothalamus). This triggers brain-derived signals to metabolic organs in the periphery, enhancing liver insulin sensitivity and boosting pancreatic insulin secretion into the portal vein, further stimulating hepatic insulin action. These processes together contribute to an effective suppression of hepatic glucose production. At the same time, brain-derived signals promote glucose uptake into peripheral tissues. Altogether, this ensures proper synchronisation of energy handling in various metabolic organs in the periphery in the postprandial state (Fig. 2).
Putative model of brain insulin’s role in peripheral metabolism and the impact of brain insulin resistance. (a) Presumed situation in people with an insulin-sensitive brain. Upon food intake, insulin is released from the pancreas into the bloodstream. It reaches the brain, passes the BBB in a receptor-mediated process and activates specialised neurons (e.g. in the hypothalamus). This introduces signals to the pancreas that propagate second-phase insulin secretion. More insulin is released into the portal vein and acts as a strong suppressor of hepatic glucose production. Endogenous glucose production is further suppressed by direct signals from the brain to the liver. This mechanism likely contributes to the adequate suppression of hepatic glucose output after food intake and all-together coordinates energy fluxes throughout the organism. (b) Presumed situation in people affected by brain insulin resistance. In this scenario, insulin cannot properly pass the BBB and cannot properly activate specialised neurons in the brain. Signals towards the periphery are compromised. Hence, there is no acute stimulation of pancreatic insulin secretion through brain-derived signals. Of note, chronic lack of these regulatory signals could contribute to insulin hypersecretion due to an overabundance of stimulatory non-neuronal signals. Furthermore, in brain insulin resistance, signals towards the liver and other metabolic organs are lacking. Altogether, this could contribute to an impaired suppression of hepatic glucose output in the postprandial state and to an impaired brain-derived modulation of whole-body energy fluxes. Over time, this could facilitate an unfavourable body fat distribution with visceral obesity, a key phenotype of high-risk subgroups of diabetes and prediabetes. This figure is available as part of a downloadable slideset
Overview of brain insulin action derived effects on peripheral metabolism. In response to food intake, insulin is released into the bloodstream. After passing the BBB, insulin reaches the brain where it acts in specialised areas, including the hypothalamus, frontal areas, insula and the dorsal striatum. This induces signals towards the periphery to suppress hepatic glucose production, to enhance peripheral glucose uptake into tissues (e.g. skeletal muscle and adipose tissue) and to propagate second-phase insulin secretion from the pancreas. These functions appear to be disturbed in brain insulin resistance. This figure is available as part of a downloadable slideset
Signals from the brain to metabolic organs in the periphery
Brain-to-periphery signals are likely transmitted through the autonomic nervous system [25, 70, 72, 76]. In line, brain insulin action appears to promote a transition from sympathetic to parasympathetic dominance [32, 55, 77] that suppresses endogenous glucose production and stimulates pancreatic insulin secretion [78]. Further signalling pathways may exist, potentially involving circulating factors, although traditional endocrine systems (e.g. hypothalamic–pituitary–adrenal axis) appear unaffected by the acute effects of brain insulin [32, 40, 54, 79].
In brain insulin resistance, the brain’s acute modulation of peripheral metabolism seems to be disrupted [25], possibly leading to impaired or absent brain-derived coordination of postprandial energy distribution in the body.
The relative contribution of brain-derived modulation to human postprandial glucose metabolism, compared with direct effects on peripheral organs, remains an intriguing question. The observations that nasal administration of insulin reduces the need for endogenous insulin post-meal [58] and that long-term nasal administration of lower-dose insulin decreases glucose fluctuations [80], suggest a significant role for brain insulin action in physiological glucose regulation. However, the reduced glucose excursions in the later study did not translate into reduced HbA1c levels [81]. Thus, it is still uncertain whether, and to what extent, disruptions in brain insulin circuits contribute to abnormal glucose metabolism (e.g. in type 2 diabetes).
Long-term consequences of brain insulin resistance in humans
The majority of data on the potential impact of brain insulin resistance comes from cross-sectional analyses that suggest connections to visceral obesity and metabolic diseases [25], as well as neurological and psychiatric disorders. These include neurodegeneration, Alzheimer’s disease, Parkinson’s disease and depression (reviewed in greater detail in [48, 82, 83]). However, the predictive power of these associations is restricted due to a limited number of longitudinal studies.
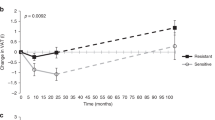
In line with findings from cross-sectional studies [84,85,86], brain insulin sensitivity is longitudinally associated with future body weight and body fat distribution [86,87,88]. Intriguingly, brain insulin sensitivity before a 24 month lifestyle intervention programme aiming to prevent type 2 diabetes predicted the programme’s effectiveness [86, 87]. Participants with brain insulin resistance struggled to lose weight or reduce their visceral fat, whereas those with good brain insulin responsiveness achieved substantial benefits. Long-term follow-up data collected 9 years afterwards indicated a lasting impact on body weight and body fat distribution, with unfavourable courses in those with brain insulin resistance [86]. Similarly, a recent trial found that brain insulin responsiveness predicted weight loss success in response to a 3 month caloric restriction in overweight, metabolically healthy adults [88].
Recent research in people at increased risk for type 2 diabetes based on detailed phenotyping has identified six unique clusters (i.e. six distinct groups of people with similar phenotypic characteristics) [89, 90]. Three clusters have a heightened risk of developing diabetes and varying risks of nephropathy, CVD and all-cause mortality, independent of blood glucose. Of note, the risk of complications is only partially connected to diabetes risk [89]. While the mechanisms driving these high-risk phenotypes remain largely unexplored, it is noteworthy that the phenotype of individuals at high risk for complications closely resembles that seen in individuals with brain insulin resistance. Therefore, brain insulin resistance may potentially be a critical factor in the pathogenesis of a phenotype with a high risk for complications of prediabetes and diabetes, a hypothesis that needs to be tested in upcoming studies.
To my knowledge, no dedicated longitudinal studies have been conducted that explore the impact of brain insulin resistance on cognitive function and mood. Nevertheless, numerous studies have investigated the impact of whole-body insulin resistance, which often overlaps with brain insulin resistance. These suggest predictive links between insulin resistance and accelerated cognitive decline [91], Alzheimer’s disease [21, 48], Parkinson’s disease [48, 82] and depression [92,93,94]. It is still being investigated whether insulin resistance is the pathomechanism or whether a common element like a proinflammatory state induces both insulin resistance and brain diseases.
Treatment of brain insulin resistance
Given the far-reaching implications on cognitive, neurological and metabolic health, brain insulin resistance is a compelling target for therapeutic intervention. However, human studies are scarce.
Two recent clinical trials that quantified brain responses to nasal insulin by fMRI demonstrated that brain insulin resistance appears to be a treatable condition. The first trial included young individuals who were overweight or obese. Despite no significant weight loss, an 8 week exercise intervention improved insulin responsiveness in the dorsal striatum (putamen) to a level similar to that seen in lean individuals [95]. The second trial evaluated pharmacological treatment with the sodium–glucose cotransporter 2 (SGLT2) inhibitor empagliflozin in individuals with prediabetes who were overweight or obese [96]. Irrespective of weight loss, SGLT2 inhibition over 8 weeks restored hypothalamic insulin sensitivity. Of note, mediation analyses indicated that this improvement in hypothalamic insulin responsiveness appears to drive the reduction of liver fat content and enhancement of fasting blood glucose levels that were also achieved with this SGLT2 inhibitor treatment [96].
In line with these findings, brain effects have been reported for dapagliflozin, another SGLT2 inhibitor [97]. While this study was focused on food-cue reactivity and did not test brain insulin responsiveness, it appears likely that empagliflozin and dapagliflozin have comparable effects [98]. While SGLT2 is expressed in the brain [99], it is unclear whether these pharmacological inhibitors act there directly or indirectly via peripheral action with subsequent projections towards the brain [98, 99].
Thiazolidinediones, a class of insulin-sensitising drugs, have not been specifically evaluated for their impact on brain insulin responsiveness in humans. Some studies have explored their effects on cognitive functions, primarily focusing on Alzheimer’s disease, yielding mixed results [21, 100]. The latest large RCT with pioglitazone was terminated early due to ineffectiveness [101]. Known side effects and the lack of cognitive benefits in dementia suggest limited potential of this substance class for treating brain insulin resistance.
Current large-scale studies are assessing the effects of glucose-lowering medications such as SGLT2 inhibitors and GLP-1 receptor agonists in neurological diseases, and may shed light on new pharmacological treatments for brain insulin resistance.
Even after significant weight loss through bariatric surgery, insulin’s effects on brain glucose uptake, as evaluated using FDG PET-CT, were not brought back to what is observed in lean individuals [102]. However, a recent study using fMRI with nasal insulin demonstrated improved brain insulin responsiveness after a 3 month low-energy diet [88]. Both imaging approaches likely capture different features of brain insulin action, highlighting the need for further research to clarify the effects of bariatric surgery and weight loss on brain insulin responsiveness.
Although evidence is growing that brain insulin resistance is in principle treatable, larger, randomised trials are necessary to confirm this and to clarify its clinical significance.
Future research directions
Even with a mounting body of evidence supporting the clinical significance of insulin action within the human brain, many open questions still remain. One of the key challenges is understanding the complex communication between the brain and peripheral organs. Unravelling the extent to which insulin-induced effects in the brain contribute to the regulation of whole-body metabolism following food intake, compared with the direct effects of insulin on target organs such as the liver or adipocytes, will be an intriguing endeavour. Clearly, more research in this direction and new non-invasive tools are necessary to decipher the mechanisms that underly each crucial step of the pancreas–brain–periphery network.
Another challenge is to uncover the regulatory processes governing insulin’s transport into the brain, as well as the precise mechanisms through which signals originating from the brain are conveyed towards target organs. Understanding whether the regulation of these mechanisms varies for each target is essential for a comprehensive understanding of the system as a whole as well as for developing organ-specific interventions.
Insulin signalling in the brain occurs physiologically in the postprandial state, a situation during which numerous signalling factors undergo dynamic fluctuations (e.g. incretin hormones and glucagon [27]). Furthermore, additional factors inform the brain about energy availability, including leptin. Determining how these factors interact with insulin in neurons and other brain cells is a largely unchartered territory and needs exploration. This will be of special importance as a number of upcoming pharmacotherapies specifically address such postprandial signalling pathways.
Further exploration is needed on how impaired brain insulin action contributes to high-risk phenotypes in prediabetes and diabetes, neurological disorders and psychiatric conditions. This could clarify the pathophysiological contribution, thereby aiding more effective prevention and intervention strategies.
A significant obstacle in assessing brain insulin resistance in larger studies and testing the potential role in clinical management is the lack of precise biomarkers. Current diagnostic procedures, such as fMRI combined with nasal administration of insulin, are costly and time-consuming. Developing easy-to-use, non-invasive tools such as biomarkers, digital tools or combinations thereof should become a priority. Such advancements could simplify diagnoses, enable accurate risk stratification and facilitate monitoring of disease progression.
Lastly, refining and optimising therapeutic methods for brain insulin resistance could open preventative or therapeutic possibilities not only for obesity and metabolic disorders but also for related neurological and psychiatric conditions.
In conclusion, exploring the role of brain insulin signalling is a thrilling and rapidly evolving research field, with implications beyond glucose metabolism. Progress in this central area requires a multidisciplinary effort to translate research findings into clinical practice and improve people’s lives.
Abbreviations
- BBB:
-
Blood–brain barrier
- CSF:
-
Cerebrospinal fluid
- FDG:
-
Fluorodeoxyglucose
- fMRI:
-
Functional MRI
- MEG:
-
Magnetoencephalography
- PET:
-
Positron emission tomography
- SGLT2:
-
Sodium–glucose cotransporter 2
References
Margolis RU, Altszuler N (1967) Insulin in the cerebrospinal fluid. Nature 215(5108):1375–1376. https://doi.org/10.1038/2151375a0
Havrankova J, Roth J, Brownstein M (1978) Insulin receptors are widely distributed in the central nervous system of the rat. Nature 272(5656):827–829. https://doi.org/10.1038/272827a0
Woods SC, Lotter EC, McKay LD, Porte D (1979) Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature 282(5738):503–505. https://doi.org/10.1038/282503a0
Woods SC (2013) Metabolic signals and food intake. Forty years of progress. Appetite 71:440–444. https://doi.org/10.1016/j.appet.2012.08.016
Porte D, Woods SC (1981) Regulation of food intake and body weight by insulin. Diabetologia 20(1):274–280. https://doi.org/10.1007/BF00254493
Brüning JC, Gautam D, Burks DJ et al (2000) Role of brain insulin receptor in control of body weight and reproduction. Science 289(5487):2122–2125. https://doi.org/10.1126/science.289.5487.2122
Obici S, Zhang BB, Karkanias G, Rossetti L (2002) Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 8(12):1376–1382. https://doi.org/10.1038/nm798
Pocai A, Lam TKT, Gutierrez-Juarez R et al (2005) Hypothalamic K(ATP) channels control hepatic glucose production. Nature 434(7036):1026–1031. https://doi.org/10.1038/nature03439
Abbott NJ, Rönnbäck L, Hansson E (2006) Astrocyte–endothelial interactions at the blood–brain barrier. Nat Rev Neurosci 7(1):41–53. https://doi.org/10.1038/nrn1824
Gray SM, Barrett EJ (2018) Insulin transport into the brain. Am J Physiol Cell Physiol 315(2):C125–C136. https://doi.org/10.1152/ajpcell.00240.2017
Rhea EM, Banks WA (2021) A historical perspective on the interactions of insulin at the blood-brain barrier. J Neuroendocrinol 33(4):e12929. https://doi.org/10.1111/jne.12929
Chen W, Cai W, Hoover B, Kahn CR (2022) Insulin action in the brain: cell types, circuits, and diseases. Trends Neurosci 45(5):384–400. https://doi.org/10.1016/j.tins.2022.03.001
Unger JW, Livingston JN, Moss AM (1991) Insulin receptors in the central nervous system: localization, signalling mechanisms and functional aspects. Prog Neurobiol 36(5):343–362. https://doi.org/10.1016/0301-0082(91)90015-S
Porniece Kumar M, Cremer AL, Klemm P et al (2021) Insulin signalling in tanycytes gates hypothalamic insulin uptake and regulation of AgRP neuron activity. Nat Metab 3(12):1662–1679. https://doi.org/10.1038/s42255-021-00499-0
Wallum BJ, Taborsky GJ, Porte D et al (1987) Cerebrospinal fluid insulin levels increase during intravenous insulin infusions in man. J Clin Endocrinol Metab 64(1):190–194. https://doi.org/10.1210/jcem-64-1-190
Csajbók ÉA, Tamás G (2016) Cerebral cortex: a target and source of insulin? Diabetologia 59(8):1609–1615. https://doi.org/10.1007/s00125-016-3996-2
Kern W, Benedict C, Schultes B et al (2006) Low cerebrospinal fluid insulin levels in obese humans. Diabetologia 49(11):2790–2792. https://doi.org/10.1007/s00125-006-0409-y
Bakker W, Imbernon M, Salinas CG et al (2022) Acute changes in systemic glycemia gate access and action of GLP-1R agonist on brain structures controlling energy homeostasis. Cell Rep 41(8):111698. https://doi.org/10.1016/j.celrep.2022.111698
Heni M, Schöpfer P, Peter A et al (2014) Evidence for altered transport of insulin across the blood-brain barrier in insulin-resistant humans. Acta Diabetol 51(4):679–681. https://doi.org/10.1007/s00592-013-0546-y
Sartorius T, Peter A, Heni M et al (2015) The brain response to peripheral insulin declines with age: a contribution of the blood-brain barrier? PloS One 10(5):e0126804. https://doi.org/10.1371/journal.pone.0126804
Kellar D, Craft S (2020) Brain insulin resistance in Alzheimer’s disease and related disorders: mechanisms and therapeutic approaches. Lancet Neurol 19(9):758–766. https://doi.org/10.1016/S1474-4422(20)30231-3
Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D (1998) Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology 50(1):164–168. https://doi.org/10.1212/WNL.50.1.164
Gil-Bea FJ, Solas M, Solomon A et al (2010) Insulin levels are decreased in the cerebrospinal fluid of women with prodomal Alzheimer’s disease. J Alzheimers Dis 22(2):405–413. https://doi.org/10.3233/JAD-2010-100795
Sagües-Sesé E, Rioja J, Garzón-Maldonado FJ, Narváez M, García-Arnés JA, García-Casares N (2022) Insulin-related biomarkers in cerebrospinal fluid in mild cognitive impairment and Alzheimer’s disease: a systematic review. J Alzheimers Dis 90(1):1–13. https://doi.org/10.3233/JAD-220688
Kullmann S, Kleinridders A, Small DM et al (2020) Central nervous pathways of insulin action in the control of metabolism and food intake. Lancet Diabetes Endocrinol 8(6):524–534. https://doi.org/10.1016/S2213-8587(20)30113-3
Hopkins DF, Williams G (1997) Insulin receptors are widely distributed in human brain and bind human and porcine insulin with equal affinity. Diabet Med 14(12):1044–1050. https://doi.org/10.1002/(SICI)1096-9136(199712)14:12<1044::AID-DIA508>3.0.CO;2-F
Theilade S, Christensen MB, Vilsbøll T, Knop FK (2021) An overview of obesity mechanisms in humans: endocrine regulation of food intake, eating behaviour and common determinants of body weight. Diabetes Obes Metab 23(Suppl 1):17–35. https://doi.org/10.1111/dom.14270
Hallschmid M (2021) Intranasal insulin. J Neuroendocrinol 33(4):e12934. https://doi.org/10.1111/jne.12934
Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL (2002) Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci 5(6):514–516. https://doi.org/10.1038/nn849
Schmid V, Kullmann S, Gfrörer W et al (2018) Safety of intranasal human insulin: a review. Diabetes Obes Metab 20(7):1563–1577. https://doi.org/10.1111/dom.13279
Nijssen KMR, Mensink RP, Joris PJ (2023) Effects of intranasal insulin administration on cerebral blood flow and cognitive performance in adults: a systematic review of randomized, placebo-controlled intervention studies. Neuroendocrinology 113(1):1–13. https://doi.org/10.1159/000526717
Kullmann S, Veit R, Peter A et al (2018) Dose-dependent effects of intranasal insulin on resting-state brain activity. J Clin Endocrinol Metab 103(1):253–262. https://doi.org/10.1210/jc.2017-01976
Tschritter O, Preissl H, Hennige AM et al (2006) The cerebrocortical response to hyperinsulinemia is reduced in overweight humans: a magnetoencephalographic study. Proc Natl Acad Sci 103(32):12103–12108. https://doi.org/10.1073/pnas.0604404103
Tschritter O, Hennige AM, Preissl H et al (2007) Cerebrocortical beta activity in overweight humans responds to insulin detemir. PloS One 2(11):e1196. https://doi.org/10.1371/journal.pone.0001196
Tschritter O, Preissl H, Hennige AM et al (2009) The insulin effect on cerebrocortical theta activity is associated with serum concentrations of saturated nonesterified fatty acids. J Clin Endocrinol Metab 94(11):4600–4607. https://doi.org/10.1210/jc.2009-0469
Tschritter O, Preissl H, Yokoyama Y, Machicao F, Häring H-U, Fritsche A (2007) Variation in the FTO gene locus is associated with cerebrocortical insulin resistance in humans. Diabetologia 50(12):2602–2603. https://doi.org/10.1007/s00125-007-0839-1
Tschritter O, Haupt A, Preissl H et al (2011) An obesity risk SNP (rs17782313) near the MC4R gene is associated with cerebrocortical insulin resistance in humans. J Obes 2011:283153. https://doi.org/10.1155/2011/283153
Ketterer C, Heni M, Stingl K et al (2014) Polymorphism rs3123554 in CNR2 reveals gender-specific effects on body weight and affects loss of body weight and cerebral insulin action. Obes Silver Spring Md 22(3):925–931. https://doi.org/10.1002/oby.20573
Rebelos E, Rinne JO, Nuutila P, Ekblad LL (2021) Brain glucose metabolism in health, obesity, and cognitive decline—does insulin have anything to do with it? A narrative review. J Clin Med 10(7):1532. https://doi.org/10.3390/jcm10071532
Kullmann S, Blum D, Jaghutriz BA et al (2021) Central insulin modulates dopamine signaling in the human striatum. J Clin Endocrinol Metab 106(10):2949–2961. https://doi.org/10.1210/clinem/dgab410
Kullmann S, Heni M, Fritsche A, Preissl H (2015) Insulin action in the human brain: evidence from neuroimaging studies. J Neuroendocrinol 27(6):419–423. https://doi.org/10.1111/jne.12254
Weinstein AM (2023) Reward, motivation and brain imaging in human healthy participants - a narrative review. Front Behav Neurosci 17:1123733. https://doi.org/10.3389/fnbeh.2023.1123733
Patel JC, Carr KD, Rice ME (2023) Actions and consequences of insulin in the striatum. Biomolecules 13(3):518. https://doi.org/10.3390/biom13030518
Heni M, Wagner R, Kullmann S et al (2017) Hypothalamic and striatal insulin action suppresses endogenous glucose production and may stimulate glucose uptake during hyperinsulinemia in lean but not in overweight men. Diabetes 66(7):1797–1806. https://doi.org/10.2337/db16-1380
Evrard HC (2019) The organization of the primate insular cortex. Front Neuroanat 13:43. https://doi.org/10.3389/fnana.2019.00043
Steuernagel L, Lam BYH, Klemm P et al (2022) HypoMap-a unified single-cell gene expression atlas of the murine hypothalamus. Nat Metab 4(10):1402–1419. https://doi.org/10.1038/s42255-022-00657-y
Hummel J, Benkendorff C, Fritsche L et al (2023) Brain insulin action on peripheral insulin sensitivity in women depends on menstrual cycle phase. Nat Metab 5(9):1475–1482. https://doi.org/10.1038/s42255-023-00869-w
Arnold SE, Arvanitakis Z, Macauley-Rambach SL et al (2018) Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol 14(3):168–181. https://doi.org/10.1038/nrneurol.2017.185
Wagner L, Veit R, Kübler C et al (2023) Brain insulin responsiveness is linked to age and peripheral insulin sensitivity. Diabetes Obes Metab 25(8):2171–2180. https://doi.org/10.1111/dom.15094
Tschritter O, Hennige AM, Preissl H et al (2009) Insulin effects on beta and theta activity in the human brain are differentially affected by ageing. Diabetologia 52(1):169–171. https://doi.org/10.1007/s00125-008-1187-5
Heni M, Kullmann S, Veit R et al (2013) Variation in the obesity risk gene FTO determines the postprandial cerebral processing of food stimuli in the prefrontal cortex. Mol Metab 3(2):109–113. https://doi.org/10.1016/j.molmet.2013.11.009
Schriever SC, Kabra DG, Pfuhlmann K et al (2020) Type 2 diabetes risk gene Dusp8 regulates hypothalamic Jnk signaling and insulin sensitivity. J Clin Invest 130(11):6093–6108. https://doi.org/10.1172/JCI136363
Wagner L, Veit R, Fritsche L et al (2002) Sex differences in central insulin action: effect of intranasal insulin on neural food cue reactivity in adults with normal weight and overweight. Int J Obes 46(9):1662–1670. https://doi.org/10.1038/s41366-022-01167-3
Heni M, Kullmann S, Ketterer C et al (2012) Nasal insulin changes peripheral insulin sensitivity simultaneously with altered activity in homeostatic and reward-related human brain regions. Diabetologia 55(6):1773–1782. https://doi.org/10.1007/s00125-012-2528-y
Heni M, Wagner R, Kullmann S et al (2014) Central insulin administration improves whole-body insulin sensitivity via hypothalamus and parasympathetic outputs in men. Diabetes 63(12):4083–4088. https://doi.org/10.2337/db14-0477
Dash S, Xiao C, Morgantini C, Koulajian K, Lewis GF (2015) Intranasal insulin suppresses endogenous glucose production in humans compared with placebo in the presence of similar venous insulin concentrations. Diabetes 64(3):766–774. https://doi.org/10.2337/db14-0685
Scherer T, Wolf P, Smajis S et al (2017) Chronic intranasal insulin does not affect hepatic lipids but lowers circulating BCAAs in healthy male subjects. J Clin Endocrinol Metab 102(4):1325–1332. https://doi.org/10.1210/jc.2016-3623
Benedict C, Brede S, Schiöth HB et al (2011) Intranasal insulin enhances postprandial thermogenesis and lowers postprandial serum insulin levels in healthy men. Diabetes 60(1):114–118. https://doi.org/10.2337/db10-0329
Kishore P, Boucai L, Zhang K et al (2011) Activation of KATP channels suppresses glucose production in humans. J Clin Invest 121(12):4916–20. https://doi.org/10.1172/JCI58035
Esterson YB, Carey M, Boucai L et al (2016) Central regulation of glucose production may be impaired in type 2 diabetes. Diabetes 65(9):2569–2579. https://doi.org/10.2337/db15-1465
Brüning JC, Fenselau H (2023) Integrative neurocircuits that control metabolism and food intake. Science 381(6665):eabl7398. https://doi.org/10.1126/science.abl7398
Lewis GF, Carpentier AC, Pereira S, Hahn M, Giacca A (2021) Direct and indirect control of hepatic glucose production by insulin. Cell Metab 33(4):709–720. https://doi.org/10.1016/j.cmet.2021.03.007
Plomgaard P, Hansen JS, Ingerslev B et al (2018) Nasal insulin administration does not affect hepatic glucose production at systemic fasting insulin levels. Diabetes Obes Metab 21(4):993–1000. https://doi.org/10.1111/dom.13615
Gancheva S, Koliaki C, Bierwagen A et al (2015) Effects of intranasal insulin on hepatic fat accumulation and energy metabolism in humans. Diabetes 64(6):1966–1975. https://doi.org/10.2337/db14-0892
Scherer T, Sakamoto K, Buettner C (2021) Brain insulin signalling in metabolic homeostasis and disease. Nat Rev Endocrinol 17(8):468–483. https://doi.org/10.1038/s41574-021-00498-x
Iwen KA, Scherer T, Heni M et al (2014) Intranasal insulin suppresses systemic but not subcutaneous lipolysis in healthy humans. J Clin Endocrinol Metab 99(2):E246-251. https://doi.org/10.1210/jc.2013-3169
Changting X, Satya D, Priska S, Lewis GF (2017) Effects of intranasal insulin on triglyceride-rich lipoprotein particle production in healthy men. Arterioscler Thromb Vasc Biol 37(9):1776–1781. https://doi.org/10.1161/ATVBAHA.117.309705
Heni M, Wagner R, Kullmann S, Preissl H, Fritsche A (2015) Response to comment on Heni et al central insulin administration improves whole-body insulin sensitivity via hypothalamus and parasympathetic outputs in men. Diabetes 2014;63:4083-4088. Diabetes 64(6):e8-9. https://doi.org/10.2337/db15-0209
Heni M, Wagner R, Willmann C et al (2020) Insulin action in the hypothalamus increases second-phase insulin secretion in humans. Neuroendocrinology 110(11–12):929–937. https://doi.org/10.1159/000504551
Borgmann D, Fenselau H (2023) Vagal pathways for systemic regulation of glucose metabolism. Semin Cell Dev Biol 156:244–252. https://doi.org/10.1016/j.semcdb.2023.07.010
Langhans W, Watts AG, Spector AC (2023) The elusive cephalic phase insulin response: triggers, mechanisms, and functions. Physiol Rev 103(2):1423–1485. https://doi.org/10.1152/physrev.00025.2022
Hampton RF, Jimenez-Gonzalez M, Stanley SA (2022) Unravelling innervation of pancreatic islets. Diabetologia 65(7):1069–1084. https://doi.org/10.1007/s00125-022-05691-9
Kullmann S, Fritsche A, Wagner R et al (2017) Hypothalamic insulin responsiveness is associated with pancreatic insulin secretion in humans. Physiol Behav 176:134–138. https://doi.org/10.1016/j.physbeh.2017.03.036
Lustig RH (2002) Hypothalamic obesity: the sixth cranial endocrinopathy. Endocrinologist 12(3):210–217. https://doi.org/10.1097/00019616-200205000-00008
Xiao C, Dash S, Stahel P, Lewis GF (2018) Effects of intranasal insulin on endogenous glucose production in insulin resistant men. Diabetes Obes Metab 20(7):1751–1754. https://doi.org/10.1111/dom.13289
Kalsbeek A, Bruinstroop E, Yi CX, Klieverik LP, La Fleur SE, Fliers E (2010) Hypothalamic control of energy metabolism via the autonomic nervous system. Ann N Y Acad Sci 1212:114–129. https://doi.org/10.1111/j.1749-6632.2010.05800.x
Ruud J, Steculorum SM, Brüning JC (2017) Neuronal control of peripheral insulin sensitivity and glucose metabolism. Nat Commun 8:15259. https://doi.org/10.1038/ncomms15259
Marino JS, Xu Y, Hill JW (2011) Central insulin and leptin-mediated autonomic control of glucose homeostasis. Trends Endocrinol Metab 22(7):275–285. https://doi.org/10.1016/j.tem.2011.03.001
Dhindsa S, Chemitiganti R, Ghanim H et al (2018) Intranasal insulin administration does not affect LH concentrations in men with diabetes. Int J Endocrinol 2018:6170154. https://doi.org/10.1155/2018/6170154
Aponte Becerra L, Galindo Mendez B, Khan F et al (2022) Safety of intranasal insulin in type 2 diabetes on systemic insulin: a double-blinded placebo-controlled sub-study of memaid trial. Arch Diabetes Obes 4(2):403–415
Becerra LA, Gavrieli A, Khan F et al (2023) Daily intranasal insulin at 40IU does not affect food intake and body composition: a placebo-controlled trial in older adults over a 24-week period with 24-weeks of follow-up. Clin Nutr 42(6):825–834. https://doi.org/10.1016/j.clnu.2023.04.008
Athauda D, Foltynie T (2016) Insulin resistance and Parkinson’s disease: a new target for disease modification? Prog Neurobiol 145–146:98–120. https://doi.org/10.1016/j.pneurobio.2016.10.001
Hamer JA, Testani D, Mansur RB, Lee Y, Subramaniapillai M, McIntyre RS (2019) Brain insulin resistance: a treatment target for cognitive impairment and anhedonia in depression. Exp Neurol 315:1–8. https://doi.org/10.1016/j.expneurol.2019.01.016
Rebelos E, Nummenmaa L, Dadson P, Latva-Rasku A, Nuutila P (2021) Brain insulin sensitivity is linked to body fat distribution-the positron emission tomography perspective. Eur J Nucl Med Mol Imaging 48(4):966–968. https://doi.org/10.1007/s00259-020-05064-7
Kullmann S, Heni M, Veit R et al (2015) Selective insulin resistance in homeostatic and cognitive control brain areas in overweight and obese adults. Diabetes Care 38(6):1044–1050. https://doi.org/10.2337/dc14-2319
Kullmann S, Valenta V, Wagner R et al (2020) Brain insulin sensitivity is linked to adiposity and body fat distribution. Nat Commun 11(1):1841. https://doi.org/10.1038/s41467-020-15686-y
Tschritter O, Preissl H, Hennige AM et al (2012) High cerebral insulin sensitivity is associated with loss of body fat during lifestyle intervention. Diabetologia 55(1):175–182. https://doi.org/10.1007/s00125-011-2309-z
Tiedemann LJ, Meyhöfer SM, Francke P, Beck J, Büchel C, Brassen S (2022) Insulin sensitivity in mesolimbic pathways predicts and improves with weight loss in older dieters. eLife 11:e76835. https://doi.org/10.7554/eLife.76835
Wagner R, Heni M, Tabák AG et al (2021) Pathophysiology-based subphenotyping of individuals at elevated risk for type 2 diabetes. Nat Med 27(1):49–57. https://doi.org/10.1038/s41591-020-1116-9
Wagner R, Heni M, Kantartzis K et al (2022) Lower hepatic fat is associated with improved insulin secretion in a high-risk prediabetes subphenotype during lifestyle intervention. Diabetes 72(3):362–366. https://doi.org/10.2337/db22-0441
Willmann C, Brockmann K, Wagner R et al (2020) Insulin sensitivity predicts cognitive decline in individuals with prediabetes. BMJ Open Diabetes Res Care 8(2):e001741. https://doi.org/10.1136/bmjdrc-2020-001741
Ford AH, Flicker L, Hankey GJ et al (2015) Insulin resistance and depressive symptoms in older men: the health in men study. Am J Geriatr Psychiatry 23(8):872–880. https://doi.org/10.1016/j.jagp.2014.10.010
Mezuk B, Eaton WW, Albrecht S, Golden SH (2008) Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care 31(12):2383–2390. https://doi.org/10.2337/dc08-0985
Watson KT, Simard JF, Henderson VW et al (2021) Incident major depressive disorder predicted by three measures of insulin resistance: a Dutch cohort study. Am J Psychiatry 178(10):914–920. https://doi.org/10.1176/appi.ajp.2021.20101479
Kullmann S, Goj T, Veit R et al (2022) Exercise restores brain insulin sensitivity in sedentary adults who are overweight and obese. JCI Insight 7(18):e161498. https://doi.org/10.1172/jci.insight.161498
Kullmann S, Hummel J, Wagner R et al (2021) Empagliflozin improves insulin sensitivity of the hypothalamus in humans with prediabetes: a randomized, double-blind, placebo-controlled, phase 2 trial. Diabetes Care 45(2):398–406. https://doi.org/10.2337/dc21-1136
van Ruiten CC, Veltman DJ, Schrantee A et al (2022) Effects of dapagliflozin and combination therapy with exenatide on food-cue induced brain activation in patients with type 2 diabetes. J Clin Endocrinol Metab 107(6):e2590–e2599. https://doi.org/10.1210/clinem/dgac043
Hummel J, Kullmann S, Heni M (2022) Spotlight on the human brain: central actions of SGLT2 inhibitors? J Clin Endocrinol Metab 107(7):e3080–e3081. https://doi.org/10.1210/clinem/dgac179
Pawlos A, Broncel M, Woźniak E, Gorzelak-Pabiś P (2021) Neuroprotective effect of SGLT2 inhibitors. Mol Basel Switz 26(23):7213. https://doi.org/10.3390/molecules26237213
Michailidis M, Tata DA, Moraitou D et al (2022) Antidiabetic drugs in the treatment of Alzheimer’s disease. Int J Mol Sci 23(9):4641. https://doi.org/10.3390/ijms23094641
Burns DK, Alexander RC, Welsh-Bohmer KA et al (2021) Safety and efficacy of pioglitazone for the delay of cognitive impairment in people at risk of Alzheimer’s disease (TOMMORROW): a prognostic biomarker study and a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Neurol 20(7):537–547. https://doi.org/10.1016/S1474-4422(21)00043-0
Rebelos E, Immonen H, Bucci M et al (2019) Brain glucose uptake is associated with endogenous glucose production in obese patients before and after bariatric surgery and predicts metabolic outcome at follow-up. Diabetes Obes Metab 21(2):218–226. https://doi.org/10.1111/dom.13501
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Acknowledgements
This review is based on my Minkowski lecture and therefore is without co-authors. However, I am very grateful for the support of many colleagues and deeply honoured to have received this important award. My scientific work on insulin and the human brain would not have been possible without the dedication, support and important insights from many colleagues and collaborators, and particularly the commitment of our study participants. I am deeply grateful for this invaluable support. In particular, I wish to extend my heartfelt appreciation to H.-U. Häring (University of Tübingen), my mentor and guiding force throughout this scientific journey. His support and enthusiasm for research ignited my own interest, and his mentorship has been instrumental in shaping the scope and quality of my work. My friend and colleague, R. Wagner (DDZ, Düsseldorf), deserves a special acknowledgement for his consistent support and countless intellectually stimulating scientific discussions. His insights and perspectives have been indispensable to the progression and success of my research. A sincere acknowledgement goes to S. Kullmann (University of Tübingen), with whom I had the honour of having an excellent and highly productive long-term collaboration. Her neuroimaging expertise played a pivotal role in many key projects on insulin and the brain. I am profoundly grateful to A. Fritsche (University of Tübingen) for countless enlightening discussions and for teaching me many innovative techniques for clinical research in diabetes. In addition, A. Peter’s (University of Tübingen) contributions cannot be understated. His assistance and expertise in the analytical aspects of metabolic research have enhanced my understanding and enriched the depth of my scientific work. Lastly, my deep appreciation goes to J. Hummel (Ulm University) and L. Fritsche (University of Tübingen). Their invaluable help in organising and analysing our clinical studies was integral to the seamlessness of our research. In sum, my deepest gratitude goes to all who have contributed to my scientific work.
Funding
Work in the author’s laboratory is supported by Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) - Projektnummer 518749683.
Author’s relationships and activities
MH reports research grants from Boehringer Ingelheim and Sanofi (both to the University Hospital of Tübingen) and lecture fees from Amryt, Boehringer Ingelheim, Lilly, Novartis, Novo Nordisk and Sanofi. He also served on advisory boards for Boehringer Ingelheim, Sanofi and Amryt.
Contribution statement
The author was the sole contributor to this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This review was invited based on the Minkowski lecture at the EASD, September 2022.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Heni, M. The insulin resistant brain: impact on whole-body metabolism and body fat distribution. Diabetologia 67, 1181–1191 (2024). https://doi.org/10.1007/s00125-024-06104-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-024-06104-9