Abstract
Aims/hypothesis
We examined the association of attainment of diabetes remission in the context of a 12 year intensive lifestyle intervention with subsequent incidence of chronic kidney disease (CKD) and CVD.
Methods
The Look AHEAD study was a multi-centre RCT comparing the effect of a 12 year intensive lifestyle intervention with that of diabetes support and education on CVD and other long-term health conditions. We compared the incidence of CVD and CKD among 4402 and 4132 participants, respectively, based on achievement and duration of diabetes remission. Participants were 58% female, and had a mean age of 59 years, a duration of diabetes of 6 year and BMI of 35.8 kg/m2. We applied an epidemiological definition of remission: taking no diabetes medications and having HbA1c <48 mmol/mol (6.5%) at a single point in time. We defined high-risk or very high-risk CKD based on the Kidney Disease Improving Global Outcomes (KDIGO) criteria, and CVD incidence as any occurrence of non-fatal acute myocardial infarction, stroke, admission for angina or CVD death.
Results
Participants with evidence of any remission during follow-up had a 33% lower rate of CKD (HR 0.67; 95% CI 0.52, 0.87) and a 40% lower rate of the composite CVD measure (HR 0.60; 95% CI 0.47, 0.79) in multivariate analyses adjusting for HbA1c, BP, lipid levels, CVD history, diabetes duration and intervention arm, compared with participants without remission. The magnitude of risk reduction was greatest for participants with evidence of longer-term remission.
Conclusions/interpretation
Participants with type 2 diabetes with evidence of remission had a substantially lower incidence of CKD and CVD, respectively, compared with participants who did not achieve remission. This association may be affected by post-baseline improvements in weight, fitness, HbA1c and LDL-cholesterol.
Trial registration
ClinicalTrials.gov NCT00017953
Data availability
https://repository.niddk.nih.gov/studies/look-ahead/
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Remission from type 2 diabetes is increasingly seen as an attainable goal for many patients, with potential benefits in terms of long-term morbidity, quality of life and avoidance of further pancreatic beta cell failure [1,2,3]. Although remission from diabetes has usually been associated with bariatric surgery, lifestyle interventions have been shown to be effective in achieving remission from diabetes as well as prediabetes [4,5,6,7,8]. In the Look AHEAD study (ClinicalTrials.gov NCT00017953), 12% of all intervention participants, and 21% of those with fewer than 2 years of diabetes duration, achieved remission the first year, while 10% achieved 2 years of remission [4]. However, the prevalence of diabetes remission declined with each year of follow-up, such that by year 4, only 7% remained in remission. More recently, the cluster randomised DiRECT (Diabetes Remission Clinical Trial) trial in the UK implemented an even more intensive weight loss programme with low-carbohydrate meal replacements, and observed remission incidences of 46% and 36% over 1 and 2 years, respectively, among participants with diabetes of up to 6 years of duration [5, 8]. While the DiRECT trial is now being followed by a national evaluation to determine its viability as a primary care referral option for the UK National Health Service, understanding its long-term impact remains incomplete [9].
Remission from diabetes after bariatric surgery has been shown to be followed by lower incidences of CVD and chronic kidney disease (CKD) [7, 10,11,12,13]. However, bariatric surgery leads to substantially greater and longer-term normalisation of glucose than remission achieved through lifestyle intervention [7, 10,11,12,13]. Despite the growing interest in diabetes remission as a goal through lifestyle intervention, the long-term impact of achieving this goal remains unclear. Participants in the US Diabetes Prevention Program and the Da Qing Diabetes Prevention Follow-up Study who achieved reversal from prediabetes to normal glucose tolerance showed large improvements in CVD risk factors [14, 15]. Similarly, in an observational cohort of adults with diabetes in Southern England, participants who achieved remission had a 20–40% lower incidence of CVD and microvascular disease outcomes compared with participants who did not experience remission [16]. However, to our knowledge, no studies have yet examined the impact of diabetes remission in the context of lifestyle intervention on long-term health outcomes.
As the largest and longest trial of intensive weight loss ever conducted, Look AHEAD is a unique opportunity to study the long-term effects of remission on long-term metabolic control and risk of diabetes-related complications in the context of a long-term lifestyle intervention [17]. We conducted post hoc analyses of the Look AHEAD study to examine the incidence of apparent remission from type 2 diabetes in the overall study cohort and according to intervention status over a period of 12 years (median 10.2 years) from baseline. We examined whether diabetes remission in the context of a lifestyle intervention trial results in a reduction in the incidence of diabetes-related CKD and CVD.
Methods
Study design and study population
The Look AHEAD study was a multi-centre RCT that compared the effect of a 12 year intensive lifestyle intervention (ILI) with that of diabetes support and education (DSE) on CVD and other long-term health conditions [17, 18]. We conducted an observational post hoc analysis of participants in both groups, classified them based on remission status, and then compared long-term outcomes (described below) based on any remission, and the duration of remission, over a period of 12 years. These analyses were based on the core Look AHEAD dataset, with no additional data collected for these analyses.
The study recruited and randomised 5145 adults with overweight or obesity (BMI ≥25 kg/m2 for non-insulin users or BMI ≥27 kg/m2 for insulin users) aged 45–76 years with type 2 diabetes. Sex was classified based on self-report. For the purposes of study inclusion, diabetes was identified by self-reporting, with verification of at least one of the following: (1) participant self-report that glucose-lowering drugs are being taken to lower blood sugar; (2) written or verbal confirmation from the individual's physician that the participant has type 2 diabetes; (3) fasting plasma glucose value of at least 7.0 mmol/l confirmed on a subsequent day as per ADA criteria; or (4) a statement in the participant's medical record of type 2 diabetes or laboratory results that meet ADA criteria.
Exclusion criteria included an inability to walk two blocks, a lower limb amputation for non-traumatic causes, urine dipstick protein of 4+ (equivalent to approximately >1 g protein/day), serum creatinine > 124 μmol/l in women or > 133 μmol/l in men, or present treatment with dialysis, very poorly controlled HbA1c (>11%), systolic BP >160 mmHg or diastolic BP >100) mmHg, triacylglycerol levels >6.8 mmol/l, or poor functional status, defined by an inability to complete a graded exercise test.
Intervention
Details of the ILI have been provided previously [18, 19]. It included weekly group and individual sessions in the first 6 months, followed by two group sessions and one individual session per month for the second 6 months, and two contacts per month (at least one in person) for years 2–4. From years 4–12, participants were encouraged to attend monthly support sessions. The multi-component ILI aimed to reduce total caloric intake to 5021 to 7532 kJ/day (1200 to 1800 kcal/day) based on initial weight, reduce total fat and saturated fat intake to less than 30% and 10%, respectively, and increase physical activity to a level of 175 min per week using brisk walking and other moderate-intensity activities. Behavioural strategies, including self-monitoring and problem solving, were used to assist in meeting behavioural, weight, dietary and physical activity goals. Liquid meal replacements were provided in the first year to assist in meeting dietary goals.
Participants receiving DSE were offered three group sessions each year, focusing on diet, physical activity and social support, but individualised behavioural support was not provided. During periods of rapid weight loss, participants monitored blood sugar so that Look AHEAD medical staff could determine whether reductions in diabetes medications were needed to reduce risk of hypoglycaemia. However, medical or pharmacological care for control of hyperglycaemia, lipids and BP were provided by the participants’ physicians independently of the Look AHEAD study for both groups, as were any referrals or recommendations to undergo bariatric surgery.
Assessments and outcomes
Participants attended a baseline clinic visit between August 2001 and April 2004, and annual follow-up visits for 4 years, followed by visits every 2 years thereafter for 12 years. At each visit, study personnel assessed the medications being taken, health status by questionnaire, body weight using a digital scale, height using a stadiometer, and HbA1c via venous phlebotomy. Values for HbA1c, serum creatinine, and urinary albumin and creatinine were collected annually until year 4 and every 2 years thereafter. We defined remission of diabetes as a transition from meeting diabetes criteria to a prediabetes level (HbA1c <48 mmol/mol, or 6.5%) with no use of glucose-lowering medications at each particular visit. As we did not perform a follow-up HbA1c measurement within 3 months, as recommended by International Consensus, this definition should be considered an epidemiological surrogate definition of remission [1]. Further, we lacked data on the specific dates of cessation of medications or change in glucose levels, and thus quantified duration of remission in terms of the number of visits at which the participant met the remission definition. Thus, for a person with remission at one visit, the expected duration ranged from 0 to <1 year, and for a person with remission at two visits, the duration ranges from >1 to <3 years, while remission at three visits represents a range from >2 to <4 years.
We examined two primary health outcomes. The first outcome was the incidence of high-risk or very high-risk CKD based on the Kidney Disease Improving Global Outcomes (KDIGO) criteria, defined by (a) an eGFR less than 45 ml/min per 1.73 m2 regardless of the urine albumin to creatinine ratio; (b) eGFR less than 60 ml/min per 1.73 m2 and a urine albumin to creatinine ratio of at least 30 mg albumin per g creatinine; or (c) any eGFR level with a urine albumin to creatinine ratio greater than 300 mg albumin per g creatinine. The second outcome was the incidence of composite CVD using pre-specified primary outcomes (CVD death, non-fatal acute myocardial infarction, non-fatal stroke or admission for angina). These outcomes were selected because they represent long-term health impacts of remission through recognised causal (biological and treatment-related) pathways: renal disease is an indicator of the microvascular impact of extended high levels of glucose, and composite CVD is an indicator of the macrovascular impact. CKD incidence was based on data from the clinic visits, and data regarding CVD were determined through an adjudication process, as previously described [17, 20].
Statistical analyses
Descriptive statistics were used to examine the yearly prevalence of any remission (HbA1c <48 mmol/mol, or 6.5%, without use of glucose-lowering drugs) and the distribution of the number of visits at which this status was attained in the overall cohort and by intervention group. We used χ2 tests and ANOVA to compare demographic and health status characteristics and 1-year and 4-year changes in risk factors according to remission group. Based on inspection of the distribution of the number of visits at which any remission was achieved, we categorised participants into four groups: no remission, one visit with documented remission, two or three visits with remission and at least four visits with remission. For analyses stratified by intervention group, we further collapsed these into three groups (no remission, one visit with documented remission, two or more visits with remission) to allow sufficient precision of estimates. We calculated incidence rates as events divided by person-years. Our primary analyses used Cox proportional hazards regression to examine the hazard ratios between participants with no remission, remission at one visit, remission at two or three visits and remission at four visits, and adjusted for baseline age, BP, CVD history, HbA1c, years of diabetes and intervention status. Only remissions occurring prior to the events were considered in these analyses. Thus, if someone had an event prior to remission, the event would be counted and attributed to the group with no remission. Further follow-up data for remission from that point forward were not considered in the analysis of time until the first CKD/CVD event.
For additional perspective, we performed analyses in which the DSE group was treated as the referent group and their outcomes were compared with those of ILI participants with no remission, or one, two, three and >4 visits with documented remission. A two-sided p value < 0.05 was considered statistically significant.
Results
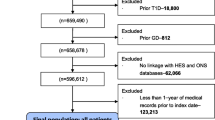
Of the 5145 participants, we further excluded 296 (5.8%) who had already met our definition of remission at baseline, 107 (2.1%) with inadequate follow-up data to estimate remission over time, and 254 (4.9%) who had bariatric surgery over the course of the study, leaving an analytic sample size of 4488 individuals.
Among the analytic sample, 12.7% met our definition of remission for at least one follow-up visit. For the ILI group, the prevalence of remission was 11.2% at year 1 and declined about 0.7 percentage points every year, while that in the DSE group remained at approximately 2%, such that, by year 4, the prevalence of any remission was 3.5 times as high in the ILI group (7.2%) compared with the DSE group (2.1%), and, by year 12, the prevalence was approximately twice as high in the ILI group (3.7% vs 1.95%) (Fig. 1).
Remission status and remission duration were each significantly associated with baseline medication use, duration of disease and levels of HbA1c, fasting plasma glucose and systolic BP (Table 1). Participants who achieved remission at some point were more likely to have been taking no medications at baseline (19% to 38% across remission groups, vs 5% in those with no remission); participants who achieved remission for at least two visits were also less likely to have had prior CVD, and remission of any duration was associated with lower baseline levels of HbA1c, fasting glucose and systolic BP but not hyperlipidaemia. Longer periods of remission were also associated with shorter duration of diabetes, ranging from 6.0 to 2.0 years of diabetes across those with no remission to those with remission for at least four visits.
Risk factor changes by remission status
Remission was also significantly associated with the changes in weight and risk factors over years 0 to 4 (Table 1). Whereas the mean weight loss after 1 and 4 years was 4 and 2.2 kg, respectively, for participants without remission, those with remission for at least one visit had lost 7.3 and 4.5 kg of body weight at years 1 and 4, respectively, and those with remission for at least four visits had lost 12.3 and 9.6 kg of body weight. There were also significantly greater improvements in HDL-cholesterol and fitness after 1 and 4 years, and significantly greater systolic BP improvements after 1 year among participants with remission compared with those without remission. Systolic BP decreased more and HDL-cholesterol increased more among participants who achieved a greater duration of diabetes remission. In contrast to the other risk factors, a smaller reduction in LDL-cholesterol was observed for those who achieved remission. In analyses stratified by intervention condition, we observed similar associations of remission status with baseline risk factors and short-term changes, except that the association of weight loss with duration of remission was stronger among ILI participants than among DSE participants (see electronic supplementary material [ESM] Table 1).
Primary outcomes
Compared with participants who did not achieve remission, participants who experienced any remission had a 33% lower rate of CKD (HR 0.67; 95% CI 0.52, 0.87) and a 40% lower rate of CVD (HR 0.60; 95% CI 0.47, 0.79) in multivariate analyses (Table 2 and Fig. 2). This association had an underlying dose–response relationship, with a notable difference between those who had no remission and any remission, and the rates of CKD and CVD each being lowest among participants who had remission for at least four visits (HR 0.45; 95% CI 0.25, 0.82 for CKD; HR 0.51; 95% CI 0.30, 0.89 for CVD).
In analyses treating DSE as the referent group (irrespective of remission status), participants in the ILI group with any remission had a 40% lower rate of CKD (HR 0.60; 95% CI 0.44, 0.82) and a 23% lower rate of CVD (HR 0.77; 95% CI 0.58, 1.03) (ESM Table 2). Participants in the ILI group with remission for at least four visits had a 60% lower rate of CKD (HR 0.40; 95% CI 0.21, 0.78) and a 35% lower rate of CVD (HR 0.65; 95% CI 0.37, 1.13).
Tests of interactions between study arm by remission status were not significant for CKD (p=0.50 and p=0.43 in crude and multivariate analyses, respectively), but were significant for CVD (p=0.04 and p=0.02 for crude and multivariate analyses, respectively). In multivariate analyses stratified by randomisation group, remission was associated with a 34% reduction in CKD (HR 0.66; 95% CI 0.48, 0.91) but not CVD (HR 0.78; 95% CI 0.58, 1.05) (Table 3) among those assigned to the ILI group. Among those in the DSE group, remission was significantly associated with a 68% reduction in CVD (HR 0.32; 95% CI 0.18, 0.59) but was not associated with a reduction in CKD (HR 0.70; 95% CI 0.45, 1.10).
Discussion
In this long-term follow-up of participants in the Look AHEAD study, we observed three main findings related to the implications of achieving diabetes remission. First, although 11% of intervention participants achieved remission at year 1 of follow-up, the percentage of participants with remission had decreased to 4% by the 8th year of the study. Second, despite the relatively short-lived durations of most episodes of remission, we found that any achievement of remission was associated with 33% and 40% lower rates of CKD and CVD, respectively, compared with participants who did not achieve remission, and risk reduction was even greater (55% and 49%, respectively) among those who had evidence of at least 4 years of remission. Third, participants with a short duration of diabetes, low starting HbA1c and a large magnitude of weight loss were most likely to experience remission.
Remission from type 2 diabetes may be associated with lower rates of CKD, CVD and other long-term health outcomes through several pathways. First, the sustained reduction in HbA1c and insulin resistance may benefit the vascular endothelium, microcirculation and reduce atherosclerosis progression [21]. Second, remission benefits may occur through the diverse physiological effects of extensive weight loss, including reductions in hyperglycaemia, BP, insulin resistance, inflammation and hepatic fat levels [2, 22]. In these analyses, participants with any remission had a slightly greater net weight loss than those without remission (2%), but those with extended remission had an 8% greater weight loss. Previous post hoc cohort analyses of the Look AHEAD study found that participants who met the weight loss goal of 10% at 1 year had a 20% lower incidence of the primary CVD outcome despite the overall null finding of the Look AHEAD intervention [17, 23]. Third, the behavioural changes associated with greater weight loss and intervention adherence, including better dietary quality, increased physical activity and higher attained physical fitness, may drive further health benefits. Finally, the achievement of remission may be a marker for other unmeasured differences or advantages arising from care and risk factor management. The observational study design limits inferences about which of these mechanisms of action account for the long-term benefit.
Compared with our study, the DiRECT study and the DIADEM-I study (Diabetes Intervention Accentuating Diet and Enhancing Metabolism) found considerably greater remission and generally similar weight loss over the first 2 years [4, 5, 24]. The DiRECT study found remission rates of 46% and 36% among intervention participants at 1 and 2 years, respectively (compared with 4% and 3% of control participants), accompanying 9.5% and 5.3% net weight losses, respectively, after 1 and 2 years [5, 25]. The DIADEM-I study reported a net 1-year remission rate of 61% (compared with 12% of control participants) and a 6.1% net weight loss [24]. By comparison, the Look AHEAD study reported 12% and 10% remission rates years 1 and 2, respectively, and a 1-year net weight loss of 8%. However, the DiRECT and DIADEM-I studies differed from the Look AHEAD study in other ways. Both studies employed total diet replacement, with targets of approximately 800–850 kcal/day, and importantly, employed an active protocol-driven approach to medication removal. After 6 months, the DiRECT study re-introduced food [5] and provided advice to increase physical activity during the maintenance period [26]. Several other studies have observed similarly profound effects on HbA1c with dietary replacement and intensive weight loss over a shorter time period than the DiRECT and Look AHEAD Studies [27,28,29,30]. In contrast, the Look AHEAD study aimed for diets of 1200–800 kcal to achieve long-term weight loss, and passively relied upon primary care providers to remove medications as appropriate [19]. The Look AHEAD study was also distinct in terms of its relatively strong emphasis on physical activity, the longer duration of diabetes at enrolment of participants (mean 6 years), and the inclusion of people with prior coronary heart disease. We are aware of only one study examining long-term outcomes following remission in non-surgical settings: a 7-year follow-up of a cohort of adults with diabetes in Southern England that found a 20–40% lower incidence of CVD and microvascular disease outcomes among participants who experienced diabetes remission, but did not collect information on intervention participation [16].
Collectively, the results of these trials suggest that remission in people with recently diagnosed type 2 diabetes is achievable through lifestyle intervention resulting in substantial weight loss, but that its effectiveness may depend upon a strong response to interventions in selected subgroups of participants. Previous analyses of Look AHEAD data showed that the percentage of participants achieving remission was twice as great in participants with a 1-year weight loss >6.5% and <2 years diabetes duration, and about 50% greater among participants with HbA1c <48 mmol/mol (6.5%) or in the top tertile of fitness change [4]. In the DiRECT study, duration of diabetes did not predict remission probability within the trial, but the average duration was only 3 years and all participants had a duration of fewer than 6 years [5, 8, 31]. Further, in post hoc analyses, weight loss and programme attendance were the strongest predictors of remission [31].
Observational studies have shown that rates of diabetes remission in the community in the absence of intensive interventions are particularly low [32,33,34]. In the UK National Diabetes Audit, which did not assess intentional weight loss, remission was only 1% in the overall population with diabetes and 3% in those with a recent diagnosis, and 8% among the subset with a large weight loss [34]. Achievement of and duration of remission were associated with several differences at baseline, including shorter duration of diabetes, higher levels of education, and lower starting HbA1c, fasting glucose levels and systolic BP. Our analyses adjusted for these factors and thus probably did not confound the association of remission with long-term outcomes. However, these differences may indicate that people who are earlier in the natural history of diabetes are more likely to achieve and benefit from attempts at remission. Our observation of higher LDL-cholesterol levels in participants achieving remission is surprising, but may be a reflection of the higher statin use among participants in the DSE group, as observed in the primary trial.
There are several limitations to these analyses. First, we lacked the necessary data to directly replicate the newly recommended clinical definition of remission, and instead relied upon an epidemiological definition as a proxy [1]. Second, we lacked data to pinpoint the onset of diabetes remission with precision, and instead used the number of visits with remission as a proxy for time without remission, thus underestimating the time in remission for some and overestimating it for others, and increasing the error variance in the estimates of risk reduction. Third, our analyses excluded approximately 10% of the overall sample due to the participants having bariatric surgery, inadequate data or already meeting the remission definition at baseline. Fourth, because the study did not have a randomised design, it is also possible that participants who achieved remission sought more intensive care and risk factor management, that drove the reduction in outcomes. Thus, there may be additional unmeasured differences in the characteristics of participants who achieved remission that also correlate with better health outcomes. Fifth, we lacked the power to stratify our core analyses by intervention status. Remission also occurred in DSE participants, albeit less frequently (7%, compared with vs 22% in ILI participants), and accounted for about one third of those with remission. Finally, we lacked the necessary data to determine which factors explained the association of remission with reductions in the incidence of CKD and CVD, including whether sex played a role or whether findings varied by sex. We speculate that remission in the DSE group was driven by similar lifestyle and risk factor changes to the factors driving remission in the intervention group, but our study was not adequately powered to examine predictors of remission within the DSE group, as only 11 CVD events and 20 CKD events occurred in DSE participants with diabetes remission. Finally, although the magnitude of weight loss appears to be a key driver in the reduction in CKD and CVD risk, whether targeting remission to influence subsequent CKD and CVD risk is more effective than targeting optimal management of the risk factors themselves cannot be tested by these analyses.
Despite the promising efficacy and outcomes of lifestyle-based remission in this and other studies, the viability of focusing on remission with lifestyle interventions as a major priority for clinical and public health efforts remains undetermined. On the one hand, these findings may drive a paradigm shift whereby selected subsets of the population are actively encouraged to strive beyond prevention of diabetes and its complications, to regression in risk status, in order to optimise long-term health outcomes [14, 15]. On the other hand, the long-term sustainability of such intensive interventions is unclear, and their incremental benefit above and beyond what may be achieved by targeting optimal risk factor management and more modest weight loss has not been tested in experimental settings. These questions underscore the need for continued follow-up in remission studies, as well as rigorous evaluation of real-world programmes of remission as they develop in the future.
Abbreviations
- CKD:
-
Chronic kidney disease
- DSE:
-
Diabetes support and education
- ILI:
-
Intensive lifestyle intervention
References
Riddle MC, Cefalu WT, Evans PH et al (2021) Consensus report: definition and interpretation of remission in type 2 diabetes. Diabetologia 64(11):2359–2366. https://doi.org/10.1111/dme.14669
Taylor R, Al-Mrabeh A, Sattar N (2019) Understanding the mechanisms of reversal of type 2 diabetes. Lancet Diabetes Endocrinol 7(9):726–736. https://doi.org/10.1016/S2213-8587(19)30076-2
Davies MJ, D’Alessio DA, Fradkin J et al (2018) Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 61(12):2461–2498. https://doi.org/10.1007/s00125-018-4729-5
Gregg EW, Chen H, Wagenknecht LE et al (2012) Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA 308(23):2489–2496. https://doi.org/10.1001/jama.2012.67929
Lean MEJ, Leslie WS, Barnes AC et al (2019) Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol 7(5):344–355. https://doi.org/10.1016/S2213-8587(19)30068-3
Sjostrom L, Peltonen M, Jacobson P et al (2014) Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 311(22):2297–2304. https://doi.org/10.1001/jama.2014.5988
Sheng B, Truong K, Spitler H, Zhang L, Tong X, Chen L (2017) The long-term effects of bariatric surgery on type 2 diabetes remission, microvascular and macrovascular complications, and mortality: a systematic review and meta-analysis. Obes Surg 27(10):2724–2732. https://doi.org/10.1007/s11695-017-2866-4
Lean ME, Leslie WS, Barnes AC et al (2018) Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet 391(10120):541–551. https://doi.org/10.1016/S0140-6736(17)33102-1
Diabetes UK. https://www.diabetes.org.uk/about_us/news/nhs-low-calorie-diet-remission
Sampalis JS, Sampalis F, Christou N (2006) Impact of bariatric surgery on cardiovascular and musculoskeletal morbidity. Surg Obes Relat Dis 2(6):587–591. https://doi.org/10.1016/j.soard.2006.08.006
Romeo S, Maglio C, Burza MA et al (2012) Cardiovascular events after bariatric surgery in obese subjects with type 2 diabetes. Diabetes Care 35(12):2613–2617. https://doi.org/10.2337/dc12-0193
Johnson BL, Blackhurst DW, Latham BB et al (2013) Bariatric surgery is associated with a reduction in major macrovascular and microvascular complications in moderately to severely obese patients with type 2 diabetes mellitus. J Am Coll Surg 216(4):545–556. https://doi.org/10.1016/j.jamcollsurg.2012.12.019; (discussion 56–8)
Coleman KJ, Haneuse S, Johnson E et al (2016) Long-term microvascular disease outcomes in patients with type 2 diabetes after bariatric surgery: evidence for the legacy effect of surgery. Diabetes Care 39(8):1400–1407. https://doi.org/10.2337/dc16-0194
Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE (2012) Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet 379(9833):2243–2251. https://doi.org/10.1016/S0140-6736(12)60525-X
Chen Y, Zhang P, Wang J et al (2021) Associations of progression to diabetes and regression to normal glucose tolerance with development of cardiovascular and microvascular disease among people with impaired glucose tolerance: a secondary analysis of the 30 year Da Qing Diabetes Prevention Outcome Study. Diabetologia 64(6):1279–1287. https://doi.org/10.1007/s00125-021-05401-x
Hounkpatin H, Stuart B, Farmer A, Dambha-Miller H (2021) Association of type 2 diabetes remission and risk of cardiovascular disease in pre-defined subgroups. Endocrinol Diabetes Metab 4(3):e00280. https://doi.org/10.1002/edm2.280
Wing RR, Bolin P, Brancati FL et al (2013) Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 369(2):145–154. https://doi.org/10.1056/NEJMoa1212914
Ryan DH, Espeland MA, Foster GD et al (2003) Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 24(5):610–628. https://doi.org/10.1016/s0197-2456(03)00064-3
Wadden TA, West DS, Delahanty L et al (2006) The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity(SilverSpring) 14(5):737–752. https://doi.org/10.1038/oby.2006.84
Wing RR, Bahnson JL, Bray GA et al (2010) Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus four-year results of the look AHEAD trial. Arch Int Med 170(17):1566–1575. https://doi.org/10.1001/archinternmed.2010.334
Gerstein HC, Werstuck GH (2013) Dysglycaemia, vasculopenia, and the chronic consequences of diabetes. Lancet Diabetes Endocrinol 1(1):71–78. https://doi.org/10.1016/S2213-8587(13)70025-1
Lingvay I, Sumithran P, Cohen RV, le Roux CW (2022) Obesity management as a primary treatment goal for type 2 diabetes: time to reframe the conversation. Lancet 399(10322):394–405. https://doi.org/10.1016/S0140-6736(21)01919-X
Gregg EW, Jakicic JM, Blackburn G et al (2016) Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 4(11):913–921. https://doi.org/10.1016/S2213-8587(16)30162-0
Taheri S, Zaghloul H, Chagoury O et al (2020) Effect of intensive lifestyle intervention on bodyweight and glycaemia in early type 2 diabetes (DIADEM-I): an open-label, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol 8(6):477–489. https://doi.org/10.1016/S2213-8587(20)30117-0
The Look AHEAD Research Group (2014) Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity (Silver Spring) 22(1):5–13. https://doi.org/10.1002/oby.20662
Cassidy S, Trenell M, Stefanetti RJ et al (2023) Physical activity, inactivity and sleep during the Diabetes Remission Clinical Trial (DiRECT). Diabet Med 40(3):e15010. https://doi.org/10.1111/dme.15010
Durrer C, McKelvey S, Singer J et al (2021) A randomized controlled trial of pharmacist-led therapeutic carbohydrate and energy restriction in type 2 diabetes. Nat Commun 12(1):5367. https://doi.org/10.1038/s41467-021-25667-4
Morris E, Aveyard P, Dyson P et al (2020) A food-based, low-energy, low-carbohydrate diet for people with type 2 diabetes in primary care: a randomized controlled feasibility trial. Diabetes Obes Metab 22(4):512–520. https://doi.org/10.1111/dom.13915
Sato J, Kanazawa A, Makita S et al (2017) A randomized controlled trial of 130 g/day low-carbohydrate diet in type 2 diabetes with poor glycemic control. Clin Nutr (Edinburgh, Scotland) 36(4):992–1000. https://doi.org/10.1016/j.clnu.2016.07.003
Umphonsathien M, Rattanasian P, Lokattachariya S, Suansawang W, Boonyasuppayakorn K, Khovidhunkit W (2022) Effects of intermittent very-low calorie diet on glycemic control and cardiovascular risk factors in obese patients with type 2 diabetes mellitus: a randomized controlled trial. J Diabetes Investig 13(1):156–166. https://doi.org/10.1111/jdi.13619
Thom G, Messow CM, Leslie WS et al (2021) Predictors of type 2 diabetes remission in the Diabetes Remission Clinical Trial (DiRECT). Diabet Med 38(8):e14395. https://doi.org/10.1111/dme.14395
Captieux M, Fleetwood K, Kennon B et al (2021) Epidemiology of type 2 diabetes remission in Scotland in 2019: a cross-sectional population-based study. PLoS Med 18(11):e1003828. https://doi.org/10.1371/journal.pmed.1003828
Karter AJ, Nundy S, Parker MM, Moffet HH, Huang ES (2014) Incidence of remission in adults with type 2 diabetes: the diabetes & aging study. Diabetes Care 37(12):3188–3195. https://doi.org/10.2337/dc14-0874
Holman N, Wild SH, Khunti K et al (2022) Incidence and characteristics of remission of type 2 diabetes in England: a cohort study using the national diabetes audit. Diabetes Care 45(5):1151–1161. https://doi.org/10.2337/dc21-2136
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Data availability
Look AHEAD data are available on request from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) data repository (https://repository.niddk.nih.gov/studies/look-ahead/).
Funding
The Look AHEAD study was funded by the National Institutes of Health through cooperative agreements with the National Institute of Diabetes and Digestive and Kidney Diseases (DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135 and DK56992) Additional funding was provided by the National Heart, Lung and Blood Institute, the National Institute of Nursing Research, the National Center on Minority Health and Health Disparities, the NIH Office of Research on Women’s Health and the US Centers for Disease Control and Prevention. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service provided personnel, medical oversight and use of facilities. The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the Indian Health Service or other funding sources. Additional support was received from the Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01RR02719), the Massachusetts General Hospital Mallinckrodt General Clinical Research Center and the Massachusetts Institute of Technology General Clinical Research Center (M01RR01066), the Harvard Clinical and Translational Science Center (RR025758-04), the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520), the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140), the University of Pittsburgh General Clinical Research Center (GCRC) (M01RR000056), the Clinical Translational Research Center funded by Clinical & Translational Science Award UL1 RR 024153 and NIH grant DK 046204, the VA Puget Sound Health Care System Medical Research Service, Department of Veterans Affairs and the Frederic C. Bartter General Clinical Research Center (M01RR01346). The following organisations have committed to make major contributions to the Look AHEAD programme: FedEx Corporation, Health Management Resources, LifeScan Inc. (a Johnson & Johnson Company), OPTIFAST® of Nestle HealthCare Nutrition Inc., Hoffmann-La Roche Inc., Abbott Nutrition and the Slim-Fast brand of Unilever North America. Some of the information contained herein was derived from data provided by the Bureau of Vital Statistics, New York City Department of Health and Mental Hygiene. EWG is supported by the Science Foundation Ireland (grant number 22/RP/10091), the UK National Institute of Health Research and the UK Royal Society.
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
EWG conceptualised and led the writing of the manuscript. HC performed the analyses. HC, MPB, RM, NM, MM and RW all contributed to researching, editing and revising key content. All authors approved the final version to be published. EWG is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
See the Electronic supplementary material (ESM) for a list of members of the Look AHEAD Study Group.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gregg, E.W., Chen, H., Bancks, M.P. et al. Impact of remission from type 2 diabetes on long-term health outcomes: findings from the Look AHEAD study. Diabetologia 67, 459–469 (2024). https://doi.org/10.1007/s00125-023-06048-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-023-06048-6