Abstract
Aims/hypothesis
The aims of this study were to identify all published prognostic models predicting retinopathy risk applicable to people with type 2 diabetes, to assess their quality and accuracy, and to validate their predictive accuracy in a head-to-head comparison using an independent type 2 diabetes cohort.
Methods
A systematic search was performed in PubMed and Embase in December 2019. Studies that met the following criteria were included: (1) the model was applicable in type 2 diabetes; (2) the outcome was retinopathy; and (3) follow-up was more than 1 year. Screening, data extraction (using the checklist for critical appraisal and data extraction for systemic reviews of prediction modelling studies [CHARMS]) and risk of bias assessment (by prediction model risk of bias assessment tool [PROBAST]) were performed independently by two reviewers. Selected models were externally validated in the large Hoorn Diabetes Care System (DCS) cohort in the Netherlands. Retinopathy risk was calculated using baseline data and compared with retinopathy incidence over 5 years. Calibration after intercept adjustment and discrimination (Harrell’s C statistic) were assessed.
Results
Twelve studies were included in the systematic review, reporting on 16 models. Outcomes ranged from referable retinopathy to blindness. Discrimination was reported in seven studies with C statistics ranging from 0.55 (95% CI 0.54, 0.56) to 0.84 (95% CI 0.78, 0.88). Five studies reported on calibration. Eight models could be compared head-to-head in the DCS cohort (N = 10,715). Most of the models underestimated retinopathy risk. Validating the models against different severities of retinopathy, C statistics ranged from 0.51 (95% CI 0.49, 0.53) to 0.89 (95% CI 0.88, 0.91).
Conclusions/interpretation
Several prognostic models can accurately predict retinopathy risk in a population-based type 2 diabetes cohort. Most of the models include easy-to-measure predictors enhancing their applicability. Tailoring retinopathy screening frequency based on accurate risk predictions may increase the efficiency and cost-effectiveness of diabetic retinopathy care.
Registration
PROSPERO registration ID CRD42018089122
Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Diabetic retinopathy is a common complication of type 2 diabetes, affecting approximately one-quarter of the type 2 diabetes population [1, 2]. Progression to sight-threatening retinopathy may cause vision loss or blindness, delivering a significant impact on quality of life [3] and high economic burden [4], although this severe end stage occurs in a small fraction of the type 2 diabetes population [2, 5]. Detecting diabetic retinopathy in its early stages through screening efforts allows for medical treatment to prevent or delay loss of vision [6]. Many countries have adopted annual or two-yearly retinopathy screening programmes for people with type 2 diabetes to ensure early diagnosis; these have been effective in reducing progression to severe stages of diabetic retinopathy [7, 8]. However, this frequency of retinopathy screening is often more than needed; most people with type 2 diabetes show no signs of retinopathy at their screening visit and do not require treatment at that time. Because of the growing prevalence of type 2 diabetes, and increased longevity of those with the disease, current annual or two-yearly retinopathy screening frequency is unsustainable, and it becomes increasingly important to manage this complication of type 2 diabetes more efficiently.
Known risk factors for the incidence or progression of diabetic retinopathy include poorly controlled HbA1c, high BP and already displaying an early stage of retinopathy [9, 10]. Based on clinical characteristics that are associated with the incidence or progression of retinopathy, several prediction models for retinopathy have been developed [11,12,13]. These models can be used to estimate the individual retinopathy risk and tailor the screening frequency accordingly, without compromising patient safety [11, 12, 14,15,16]. Adoption of tailored programmes based on these models could mitigate the burden and costs of over-screening while avoiding the risks from under-screening, thereby providing more effective and cost-effective programmes. Before adopting a prediction model into clinical practice, its accuracy must be ascertained through validation of the model in a population other than the one in which it was developed. This essential step, although often omitted, demonstrates whether the model can be generalised to other populations.
The aim of this study was to systematically review the literature to identify all studies on the development of prognostic prediction models for the risk of developing retinopathy applicable to persons with type 2 diabetes and to determine their quality and predictive accuracy. Subsequently, the selected prediction models were compared head-to-head by validating them in the same large independent population-based cohort of people with type 2 diabetes.
Methods
We performed a systematic review and an external validation study. The protocol of the systematic review was registered with the International Prospective Register of Systematic Reviews (PROSPERO) on 21 February 2018 (registration no. CRD42018089122), and the review was performed according to the PRISMA-P guideline [17]. The external validation study was reported in line with the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) guideline (see electronic supplementary material [ESM] Table 1) [18, 19].
Systematic review
Data sources and searches
The literature was systematically searched for all studies reporting on the development of a prediction model for the risk of developing retinopathy applicable to people with type 2 diabetes. PubMed and Embase were searched from inception to 10 December 2019 using the search string presented in ESM Table 2. Any additional relevant studies were identified by screening the references of the included studies by hand, and related articles were included in the screening process.
Study selection for the review
Following the guidance provided in the checklist for critical appraisal and data extraction for systematic reviews of prediction modelling studies (CHARMS) [20]) for defining the review question and study eligibility criteria, studies were eligible for inclusion when meeting the following criteria: (1) the prediction model was developed in people with type 2 diabetes or the general population including diabetes as a predictor; (2) the outcome of the prognostic model was any stage of retinopathy; and (3) the time horizon of the outcome prediction was at least 1 year. A study was excluded when meeting the following criteria: (1) it was performed in animals; (2) it was written in languages other than English or Dutch; (3) it did not address the development of a prediction model; or (4) the prediction model consisted of only one predictor.
Data extraction and quality assessment
For the included studies, data extraction was performed using CHARMS [20]. Risk of bias was assessed to identify shortcomings in study design and applicability using the prediction model risk of bias assessment tool (PROBAST) [21, 22]. A combination of two reviewers (of a group of three; A. A. van der Heijden, F. Badloe and J. W. Beulens) independently reviewed each title, abstract and full text and subsequently extracted data and assessed risk of bias and applicability of the included studies.
External validation
Cohort
For external validation of the selected (from the review) prediction models, we used data from the Hoorn Diabetes Care System (DCS) cohort. The DCS cohort is an ongoing population-based cohort including almost all people with type 2 diabetes in the catchment area of the DCS, a primary care setting in the West-Friesland area of the Netherlands [23]. As part of routine diabetes care, participants receive a standardised annual assessment including measurement of diabetes-related risk factors and complications. The resulting database included 13,955 people with type 2 diabetes in 2017, with follow-up ranging from 1 year to 19 years. The study has been approved by the Medical Ethical Review Committee of the VU University Medical Center, Amsterdam. Individuals were informed about the use of their data and were offered the opportunity to opt out. Data were used anonymously. For each individual, year of entry into the cohort was considered baseline. After exclusion of people with insufficient follow-up data on retinopathy status and exclusion of people with the specific outcome of interest at baseline, 10,715 people remained for the prediction of referable diabetic retinopathy, 10,820 for the prediction of sight-threatening retinopathy and 10,874 for the prediction of photocoagulated or proliferative retinopathy. More details can be found in the flowchart shown in ESM Fig. 1.
Predictors
According to centrally standardised protocols, examinations and clinical measurements were performed annually. Weight and height were measured when the participant was barefoot and wearing light clothes and BMI was calculated as weight (kg) / height (m2). BP was measured twice (3 min apart) after 5 min of rest in a seated position on the right arm using a random-zero sphygmomanometer (Hawksley-Gelman, Lancing, Sussex, UK); from 2003 onwards, an oscillometric device was used (Colin Press-Mate BP-8800 [Komaki City, Japan] and from 2011 onwards, a Welch Allyn ProBP 3400 [Skaneateles Falls, NY, USA]). Using fasting blood, HbA1c was assessed based on the turbidimetric inhibition immunoassay for haemolysed whole EDTA blood, blood glucose level was assessed in fluorinated plasma with the UV test using hexokinase, and levels of triacylglycerols, total cholesterol and HDL-cholesterol were determined enzymatically (Cobas c501; Roche Diagnostics, Mannheim, Germany). LDL-cholesterol concentration was calculated. From an overnight first-voided urine sample, albumin was determined by the reaction of the antigen with anti-albumin antibodies and measured turbidimetrically, and the creatinine concentration in heparinised plasma and urine was determined enzymatically (Cobas c501; Roche Diagnostics). The presence of CVD was based on self-report. Information on medication use was registered yearly by checking the dispensing labels of the medication brought to the monitoring visit. Age at diagnosis was based on registry data. Information on smoking status and ethnic background was obtained by self-report.
Retinopathy (outcome)
To determine the presence and stage of retinopathy, participants underwent a 45° fundus photograph of two retinal fields according to the EURODIAB protocol. Retinopathy stage was based on the grading of the worst eye as determined by an experienced ophthalmologist. The grading of retinopathy was performed according to the EURODIAB 0–5 scale as follows: grade 0, no retinopathy; grade 1, minimal non-proliferative retinopathy; grade 2, moderate non-proliferative retinopathy; grade 3, severe non-proliferative or pre-proliferative retinopathy; grade 4, photocoagulated retinopathy; and grade 5, proliferative retinopathy [24]. The ophthalmologist who graded the retinal images did not have access to any other clinical data from the participants. Outcomes used for validation were referable diabetic retinopathy (EURODIAB grade ≥2), sight-threatening diabetic retinopathy (EURODIAB grade ≥3) and photocoagulated or proliferative diabetic retinopathy (EURODIAB grade ≥4).
Prediction models selected for validation
Prediction models that were reported in the studies included in the systematic review were all considered for validation. The predictors and outcomes of the models were matched to the variables in the DCS cohort. When the included predictors were not available in the DCS, the predictor was replaced by a suitable proxy variable. When a proxy could not replace the predictor, the model was excluded from validation.
Statistical analysis
In the validation cohort, missing data on predictors were imputed using multiple imputation by predictive mean matching, generating five imputed datasets that were pooled using Rubin’s rules. Missing values varied from 0.4% for BMI to 4.0% for albumin/creatinine ratio; 1028 participants (9.6%) had missing data for at least one of the predictors. Baseline characteristics of the study population were presented as proportions or means with SD; variables with a skewed distribution were presented as medians with an interquartile range. For each model that could be applied to the DCS cohort, predicted retinopathy risk was calculated and compared with the incidence of each of the three retinopathy severity outcomes using a 5 year horizon. Performance of the models was estimated using calibration (agreement between predicted and observed retinopathy risk) and discrimination (the ability of the model to distinguish between those who will develop retinopathy and those who will not). Calibration was assessed by visual inspection of calibration plots, the ratio of the observed and expected retinopathy events (O/E), and the Hosmer–Lemeshow χ2 test. Calibration plots were created by plotting the observed mean retinopathy risk against the predicted mean retinopathy risk within quintiles of the predicted risk. For a well-calibrated risk, the predicted risk would be similar to the observed risk, resulting in an O/E close to 1 and the calibration slope approaching the 45° line of the plot. As most of the intercepts of the models were not reported, and recalibration of models based on the incidence of the outcome of interest in the validation population is recommended, we presented only recalibrated models based on retinopathy incidence in the DCS cohort. For each model, the mean 5 year retinopathy-free survival rate of the DCS cohort was used. Discrimination was assessed for each model using Harrell’s C statistic, accounting for the censored nature of the data. Sensitivity analyses were performed by testing model performance in people without signs of diabetic retinopathy at baseline. Furthermore, the performance of the models was tested applying a 10 year prediction horizon. All analyses were performed with the statistical software package R (version 3.6.1) (www.r-project.org) in combination with the R packages MICE (version 3.8.0) and RMS (version5.1-4).
Results
Systematic review
From the 6907 records, 103 were selected for review of the full text. Of these, 12 met our inclusion criteria, describing the development of 16 prediction models for retinopathy. The flowchart on study inclusion is presented in ESM Fig. 1.
Characteristics of the models, risk of bias, and applicability
The size of the samples used for model development of the studies included in the systematic review ranged from 295 to 454,575 (ESM Table 3). Two models used blindness as the predicted outcome while the other studies included a less severe form of retinopathy as outcome. The most commonly used prediction time horizons were 5 years and 10 years. The number of events ranged from 61 to 8063. Most of the studies used predictors routinely assessed in people with type 2 diabetes, with HbA1c as the most commonly used predictor (ESM Table 4, ESM Fig. 2).
The majority of the studies scored a high or unclear risk of bias on the analysis domain, with most models not informing or performing on the correct handling of missing data or adjustment for overfitting in model performance (ESM Table 5). The other domains were rated as low risk of bias in 75–100% of the models (ESM Fig. 3). In three studies, high concerns for applicability to our objective were scored due to selective sampling of the participants in the original studies. High applicability was scored for five of the ten studies including participants, predictors and outcomes relevant for a general type 2 diabetes population in a routine care setting.
Apparent model performance
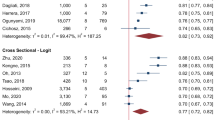
Information on calibration in the form of a plot or test was reported by four studies based on internal validation and by five studies based on external validation, all showing close agreement between predicted and observed outcomes. Six studies reported C statistics in the derivation cohorts, ranging from 0.55 (95% CI 0.54, 0.56) in Basu et al [25] to 0.90 (95% CI 0.86, 0.92) in Garcia-Finana et al [26] (Fig. 1). In four studies, discriminatory ability in an external population was reported and ranged from 0.57 (95% CI 0.51, 0.63) in Basu et al [25] to 0.84 (95% CI 0.78, 0.88) in Scanlon et al [13]. Four studies lacked information on discrimination and calibration.
External validation
Selection of models for external validation
Of the 12 studies reporting on 16 models, included in the review, eight models were excluded for external validation as the algorithm could not be extracted from the original model or the model mainly included predictors that were not available in the validation cohort and could not be approximated (ESM Table 6). For some models, predictors were approximated to enable validation. For the predictor albuminuria included in the model by Semeraro et al [27], the mean value reported for the derivation cohort was used (12 g/day). Townsend deprivation score, included in the model of Hippisley-Cox and Coupland [28], was not available in the DCS cohort and the reported sex-specific mean value of the deprivation score was used (men 0.5; women 0.8). To predict retinopathy, the model by Scanlon et al [13] used a similar linear predictor but different intercepts in people in whom none, one or two eyes were affected. We estimated the baseline hazard for developing diabetic retinopathy separately for people with and without baseline retinopathy.
Characteristics of the external validation cohort
Of the 10,874 people with type 2 diabetes at baseline who were at risk of photocoagulated or proliferative retinopathy, 83 (0.8%) reached this stage of retinopathy during the 5 year prediction horizon. Of the 10,820 people free of sight-threatening retinopathy at baseline, 144 (1.3%) developed sight-threatening retinopathy during follow-up and of the 10,715 people at risk of referable diabetic retinopathy at baseline, 237 (2.2%) developed this condition. In a population free from referable diabetic retinopathy, 493 participants (4.6%) presented with mild retinopathy at baseline. Details of the population are shown in Table 1.
Calibration
Considering the performance of the models specifically for predicting the retinopathy outcome (as they were developed for), the calibration plots display the relationship between the predicted risk and observed retinopathy incidence of the eight prediction models after recalibration based on retinopathy incidence in the DCS cohort (ESM Fig. 4, ESM Table 7). In most of the models, the first quintiles showed agreement between the predicted risk and observed retinopathy incidence, and an underestimation of retinopathy risk in people in the highest risk quintile. The calibration plots of Semeraro et al [27] and Scanlon et al [13] showed better calibration. The model of Dagliati et al [29] showed less agreement between the predicted and observed risks within lower quintiles. The O/E ratios that showed the most substantial deviation from 1 were from the models of Aspelund et al [11] and Dagliati et al [29], mainly underestimating retinopathy risk. All models showed p values <0.05 for the Hosmer–Lemeshow χ2 test.
Discrimination
In Fig. 2, the discriminatory ability of the models selected for validation is presented for each of the three retinopathy outcome measures. Considering the specific retinopathy stage used in model development, C statistics ranged from 0.51 (95% CI: 0.49, −0.53) to 0.83 (95% CI: 0.81, −0.84). Applying each model to all three retinopathy outcomes improved discriminatory power for higher severities of retinopathy in most models, and C statistics ranged from 0.51 (95% CI 0.49, 0.53) to 0.89 (95% CI 0.88, 0.91). For all three retinopathy outcomes, the models by Tanaka et al [30] Semeraro et al [27] and Aspelund et al [11] showed the highest C indices (Fig. 2).
Discriminative ability of the models for prediction of referable diabetic retinopathy (EURODIAB grade ≥2) (a), sight-threatening diabetic retinopathy (EURODIAB grade ≥3) (b) and photocoagulated or proliferative diabetic retinopathy (EURODIAB grade ≥4) (c) in the DCS cohort. Results are presented as C statistics (95% CI). The grey boxes indicate the models intended to predict the retinopathy stage shown in that figure part
Sensitivity analyses
When the prediction horizon used for the external validation was extended to 10 years, a slight decrease in performance was seen in most of the models (ESM Table 8). Applying the model in people without any signs of retinopathy at baseline resulted in the largest decrease in C statistics in the models that include baseline presence of diabetic retinopathy as a predictor (ESM Table 9).
Discussion
The current study provides a comprehensive overview of the available prognostic prediction models for developing retinopathy (beyond 1 year) in people with type 2 diabetes. We identified 12 studies describing the development of 16 prediction models with outcomes ranging from any form of retinopathy to blindness. Eight of the 16 models could be validated and directly compared head-to-head in a large independent validation cohort. Notably, we found that four models (i.e. Semeraro et al [27], Scanlon et al [13], Aspelund et al [11] and Tanaka et al [30]) showed a better calibration plus discrimination than the other four.
This systematic review demonstrated that most of the models used predictors that were obtained in routine diabetes care settings, increasing their applicability to daily practice. According to the recently published PROBAST tool, most of the models showed an overall high or unclear risk of bias, mainly caused by vague reporting on model development (domain 4). This might be due to the fact that the TRIPOD guideline for transparent reporting of prediction model studies is relatively new and was not available during the development of most of the models included in the review.
Considering the calibration of the models, even after recalibration to retinopathy incidence in the DCS cohort, most models still showed overestimation for individuals in the highest risk quintile for retinopathy. Calibration seemed best in the model of Semeraro et al [27] and Scanlon et al [13] When comparing the results of several previous external validation studies of the model of Aspelund et al [11, 14, 15], similar or slightly better discrimination was observed in the DCS cohort. External validation in the DCS cohort yielded similar results for discriminative performance after internal validation of the model of Semeraro et al [27]. For the model of Tanaka et al, better discrimination was found in the external validation compared with the models’ internal validation [30]. In this validation study, we found lower discriminatory power for the models of Scanlon et al and Hippisley-Cox and Coupland, which presented slightly lower C statistics compared with the external validation of these two models in different cohorts [13, 28]. Reasons for this lower discrimination may be the absence of some of the models’ predictor variables in the DCS cohort. The model of Scanlon et al [13] distinguishes for the presence of mild retinopathy in one or two eyes in the baseline hazard. Whether one or both eyes were affected at baseline was not recorded in the DCS data. Additionally, the discriminatory ability of Scanlon’s model improved after the exclusion of people with signs of retinopathy at baseline. The model of Hippisley-Cox and Coupland [28] used blindness as the predicted outcome; this information was not available in the DSC cohort. Photocoagulated or proliferative retinopathy was used as a proxy for this outcome. Townsend deprivation score was also not available in the DCS cohort, leaving cholesterol/HDL-cholesterol ratio, diabetes duration and presence of chronic renal disease as the predictor variables, which may have affected model performance. The model of Basu et al showed better discriminatory power after external validation in the DCS cohort, compared with previous external validation studies [25, 31]. The model of Basu et al had the highest number of predictors. Besides common predictors such as HbA1c, lipids and BP, the model includes predictors on glucose-lowering medication use, history of CVD, and renal function [25]. The model was developed using data from people with type 2 diabetes selected based on their unfavourable risk profile. Application of the model in our relatively well-controlled population showed that the model was capable of discriminating between low and high risk. For the model of Dagliati et al [29], a substantial decrease in discriminative ability was seen between the model’s internal validation and the DCS cohort. The small sample size in the original population and the low number of events might have resulted in an overfitted model that performed less well in our large cohort.
A limitation of the systematic review is that some prediction models may have been missed due to language restrictions. A limitation of the external validation study is the low incidence of retinopathy in the DCS cohort. Recent publications indicate that studies aiming to test model performance require at least 100 events [32, 33]. For referable and sight-threatening retinopathy, this criterion is fulfilled. For the more severe stages, the analysis might have been underpowered. However, we observed a stable trend in the discriminatory power with increasing severity of retinopathy, possibly indicating that the results would not be affected largely by the lower number of end-stage retinopathy events. Another limitation is that the study population was primarily of European descent, hindering the extrapolation to populations with a different migration background. The model of Scanlon et al was previously validated in cohorts with people with African Caribbean and South Asian backgrounds and showed similar accuracy as in white populations [13]. An additional limitation is that not all predictors used by the models were available in the DCS cohort.
Several study strengths should be noted. First, the results of the validation study apply to routine clinical practice settings for people with type 2 diabetes. Second, the use of a large, unselected cohort of people with type 2 diabetes, including almost all people with type 2 diabetes in the catchment area of the DCS, enhances this external validation study. Third, all predictor and outcome measurements in the cohort were performed according to centrally standardised protocols, including consistency in measuring and defining the presence and grade of retinopathy; this enhanced the reliability of the data and resulted in a low number of missing variables.
In recent literature, approaches have been suggested for reducing the frequency of unneeded retinopathy screening; these include extending the screening interval from 1 year to 2 years for people who are at low risk of retinopathy, defined by two subsequent retinopathy examinations without signs of retinopathy [34]. However, prediction models for retinopathy more efficiently use available clinical data to identify people at low risk, by providing an individual risk estimation. This study showed that several models are capable of distinguishing between those at low risk and those at high risk for retinopathy and can be used to tailor the screening frequency. Adequate performance of a model based on calibration as well as discrimination is a prerequisite for its clinical relevance. When using a model to tailor screening intervals, overestimating or underestimating the absolute risk may lead to a too frequent or infrequent screening respectively, which may be overcome by model recalibration. Once this is evaluated, clinical applicability is leading for the choice of a suitable model. Based on the results on discrimination and calibration after external validation of the models in the DCS cohort, four models performed well enough and can help facilitate tailored retinopathy care for people with type 2 diabetes. Two of these models do not include current retinopathy status as a predictor, which might seem counterintuitive from a clinical perspective. The models by Scanlon et al [13] and Aspelund et al [11] include information on current retinopathy in addition to routinely collected risk factors and predict earlier stages of retinopathy, enhancing their applicability. In the DCS we already have experience tailoring retinopathy care after implementation of the model developed by Aspelund et al. This model additionally provides a tailored screening interval ranging from 6 months to a maximum of 5 years based on the estimated retinopathy risk and hence is instrumental in adapting imaging frequency to personal risk, enhancing efficient use of medical resources. Previous studies showed that this method for personalising screening frequency is safe and reduces the average screening frequency [14,15,16].
To conclude, this comprehensive systematic review and head-to-head comparison of all existing prognostic models predicting the risk of developing retinopathy in individuals with type 2 diabetes, shows that several existing prediction models can accurately predict retinopathy risk in a population-based cohort of people with type 2 diabetes. The models of Scanlon et al [13] and Aspelund et al [11] performed best overall and contain easy-to-measure predictors, increasing their suitability for clinical practice. Tailoring retinopathy screening frequency based on individual risk predictions by these models may highly increase the efficiency of diabetic retinopathy care.
Data availability
The steering committee of the Hoorn studies will consider reasonable requests for the sharing of de-identified patient-level data. Requests should be made to the corresponding author.
Abbreviations
- CHARMS:
-
Checklist for critical appraisal and data extraction for systemic reviews of prediction modelling studies
- DCS:
-
Diabetes Care System
- O/E:
-
Ratio of the observed and expected retinopathy events
- PROBAST:
-
Prediction model risk of bias assessment tool
- TRIPOD:
-
Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis
References
Lee R, Wong TY, Sabanayagam C (2015) Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond) 2:17. https://doi.org/10.1186/s40662-015-0026-2
Mathur R, Bhaskaran K, Edwards E et al (2017) Population trends in the 10-year incidence and prevalence of diabetic retinopathy in the UK: a cohort study in the Clinical Practice Research Datalink 2004-2014. BMJ Open 7(2):e014444. https://doi.org/10.1136/bmjopen-2016-014444
Luckie R, Leese G, McAlpine R et al (2007) Fear of visual loss in patients with diabetes: results of the prevalence of diabetic eye disease in Tayside, Scotland (P-DETS) study. Diabet Med 24(10):1086–1092. https://doi.org/10.1111/j.1464-5491.2007.02180.x
Happich M, Reitberger U, Breitscheidel L, Ulbig M, Watkins J (2008) The economic burden of diabetic retinopathy in Germany in 2002. Graefes Arch Clin Exp Ophthalmol 246(1):151–159. https://doi.org/10.1007/s00417-007-0573-x
Thomas RL, Dunstan FD, Luzio SD et al (2015) Prevalence of diabetic retinopathy within a national diabetic retinopathy screening service. Br J Ophthalmol 99(1):64–68. https://doi.org/10.1136/bjophthalmol-2013-304017
Bachmann MO, Nelson SJ (1998) Impact of diabetic retinopathy screening on a British district population: case detection and blindness prevention in an evidence-based model. J Epidemiol Community Health 52(1):45–52. https://doi.org/10.1136/jech.52.1.45
Echouffo-Tcheugui JB, Ali MK, Roglic G, Hayward RA, Narayan KM (2013) Screening intervals for diabetic retinopathy and incidence of visual loss: a systematic review. Diabet Med 30(11):1272–1292. https://doi.org/10.1111/dme.12274
Broadbent D, Harding S, D’Souza YD, Peto T (2017) Screening for diabetic retinopathy in Europe - progress since 2011: national representatives meeting, Manchester. Available from http://www.drscreening2005.org.uk/manchester_2016.html. Accessed 8 Nov 2019
Stratton IM, Aldington SJ, Taylor DJ, Adler AI, Scanlon PH (2013) A simple risk stratification for time to development of sight-threatening diabetic retinopathy. Diabetes Care 36(3):580–585. https://doi.org/10.2337/dc12-0625
Zavrelova H, Hoekstra T, Alssema M et al (2011) Progression and regression: distinct developmental patterns of diabetic retinopathy in patients with type 2 diabetes treated in the diabetes care system West-Friesland, the Netherlands. Diabetes Care 34(4):867–872. https://doi.org/10.2337/dc10-1741
Aspelund T, Thornorisdottir O, Olafsdottir E et al (2011) Individual risk assessment and information technology to optimise screening frequency for diabetic retinopathy. Diabetologia 54(10):2525–2532. https://doi.org/10.1007/s00125-011-2257-7
Mehlsen J, Erlandsen M, Poulsen PL, Bek T (2012) Individualized optimization of the screening interval for diabetic retinopathy: a new model. Acta Ophthalmol 90(2):109–114. https://doi.org/10.1111/j.1755-3768.2010.01882.x
Scanlon PH, Aldington SJ, Leal J et al (2015) Development of a cost-effectiveness model for optimisation of the screening interval in diabetic retinopathy screening. Health Technol Assess 19(74):1–116. https://doi.org/10.3310/hta19740
Lund SH, Aspelund T, Kirby P et al (2016) Individualised risk assessment for diabetic retinopathy and optimisation of screening intervals: a scientific approach to reducing healthcare costs. Br J Ophthalmol 100(5):683–687. https://doi.org/10.1136/bjophthalmol-2015-307341
Soto-Pedre E, Pinies JA, Hernaez-Ortega MC (2015) External validation of a risk assessment model to adjust the frequency of eye-screening visits in patients with diabetes mellitus. J Diabetes Complicat 29(4):508–511. https://doi.org/10.1016/j.jdiacomp.2014.12.020
van der Heijden AA, Walraven I, van’t Riet E et al (2014) Validation of a model to estimate personalised screening frequency to monitor diabetic retinopathy. Diabetologia 57(7):1332–1338. https://doi.org/10.1007/s00125-014-3246-4
Shamseer L, Moher D, Clarke M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 350:g7647. https://doi.org/10.1136/bmj.g7647
Moons KG, Altman DG, Reitsma JB et al (2015) Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 162(1):W1–W73. https://doi.org/10.7326/M14-0698
Collins GS, Reitsma JB, Altman DG, Moons KG (2015) Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med 162(1):55–63. https://doi.org/10.7326/M14-0697
Moons KG, de Groot JA, Bouwmeester W et al (2014) Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med 11(10):e1001744. https://doi.org/10.1371/journal.pmed.1001744
Moons KGM, Wolff RF, Riley RD et al (2019) PROBAST: a tool to assess risk of bias and applicability of prediction model studies: explanation and elaboration. Ann Intern Med 170(1):W1–W33. https://doi.org/10.7326/M18-1377
Wolff RF, Moons KGM, Riley RD et al (2019) PROBAST: a tool to assess the risk of bias and applicability of prediction model studies. Ann Intern Med 170(1):51–58. https://doi.org/10.7326/M18-1376
van der Heijden AA, Rauh SP, Dekker JM et al (2017) The Hoorn Diabetes Care System (DCS) cohort. A prospective cohort of persons with type 2 diabetes treated in primary care in the Netherlands. BMJ Open 7(5):e015599. https://doi.org/10.1136/bmjopen-2016-015599
Aldington SJ, Kohner EM, Meuer S, Klein R, Sjolie AK (1995) Methodology for retinal photography and assessment of diabetic retinopathy: the EURODIAB IDDM complications study. Diabetologia 38(4):437–444. https://doi.org/10.1007/bf00410281
Basu S, Sussman JB, Berkowitz SA, Hayward RA, Yudkin JS (2017) Development and validation of risk equations for complications of type 2 diabetes (RECODe) using individual participant data from randomised trials. Lancet Diabetes Endocrinol 5(10):788–798. https://doi.org/10.1016/S2213-8587(17)30221-8
Garcia-Finana M, Hughes DM, Cheyne CP et al (2019) Personalized risk-based screening for diabetic retinopathy: a multivariate approach versus the use of stratification rules. Diabetes Obes Metab 21(3):560–568. https://doi.org/10.1111/dom.13552
Semeraro F, Parrinello G, Cancarini A et al (2011) Predicting the risk of diabetic retinopathy in type 2 diabetic patients. J Diabetes Complicat 25(5):292–297. https://doi.org/10.1016/j.jdiacomp.2010.12.002
Hippisley-Cox J, Coupland C (2015) Development and validation of risk prediction equations to estimate future risk of blindness and lower limb amputation in patients with diabetes: cohort study. BMJ 351:h5441. https://doi.org/10.1136/bmj.h5441
Dagliati A, Marini S, Sacchi L et al (2018) Machine learning methods to predict diabetes complications. J Diabetes Sci Technol 12(2):295–302. https://doi.org/10.1177/1932296817706375
Tanaka S, Tanaka S, Iimuro S et al (2013) Predicting macro- and microvascular complications in type 2 diabetes: the Japan Diabetes Complications Study/the Japanese Elderly Diabetes Intervention Trial risk engine. Diabetes Care 36(5):1193–1199. https://doi.org/10.2337/dc12-0958
Basu S, Sussman JB, Berkowitz SA et al (2018) Validation of risk equations for complications of type 2 diabetes (RECODe) using individual participant data from diverse longitudinal cohorts in the U.S. Diabetes Care 41(3):586–595. https://doi.org/10.2337/dc17-2002
Collins GS, Ogundimu EO, Altman DG (2016) Sample size considerations for the external validation of a multivariable prognostic model: a resampling study. Stat Med 35(2):214–226. https://doi.org/10.1002/sim.6787
Vergouwe Y, Steyerberg EW, Eijkemans MJ, Habbema JD (2005) Substantial effective sample sizes were required for external validation studies of predictive logistic regression models. J Clin Epidemiol 58(5):475–483. https://doi.org/10.1016/j.jclinepi.2004.06.017
Scanlon PH (2017) Screening intervals for diabetic retinopathy and implications for care. Curr Diab Rep 17(10):96. https://doi.org/10.1007/s11892-017-0928-6
Aspinall PA, Kinnear PR, Duncan LJ, Clarke BF (1983) Prediction of diabetic retinopathy from clinical variables and color vision data. Diabetes Care 6(2):144–148. https://doi.org/10.2337/diacare.6.2.144
Clarke PM, Gray AM, Briggs A et al (2004) A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia 47(10):1747–1759. https://doi.org/10.1007/s00125-004-1527-z
Ochs A, McGurnaghan S, Black MW et al (2019) Use of personalised risk-based screening schedules to optimise workload and sojourn time in screening programmes for diabetic retinopathy: a retrospective cohort study. PLoS Med 16(10):e1002945. https://doi.org/10.1371/journal.pmed.1002945
Acknowledgements
This study has been made possible by the collaboration with the Diabetes Care System West-Friesland. The authors thank participants and staff of the Diabetes Care System West-Friesland. Some of the data were presented as an abstract at the annual meeting of the EASD in 2017, the European Diabetes Epidemiology Group (EDEG) in 2019, and the EASD Eye Complications Study Group (EASDec) in 2019.
Funding
This research was supported by the Dutch Diabetes Research Foundation (grant no. 2014.00.1753). The funder had no role in the study design, in the collection, analysis, and interpretation of data, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
AAH and JWB were responsible for the design of the study. AAH, JWB and FB screened citations for inclusion and were involved in risk of bias assessment, data extraction and data interpretation. AAH collected, cleaned and analysed the data. All co-authors were involved in the interpretation of the data. The manuscript was written by AAH with input from all co-authors. All authors approved the final version of this manuscript. AAH is the guarantor of this manuscript and accepts full responsibility for the work and the conduct of the study, had access to the data and controlled the decision to publish.
Corresponding author
Ethics declarations
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM
(PDF 705 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van der Heijden, A.A., Nijpels, G., Badloe, F. et al. Prediction models for development of retinopathy in people with type 2 diabetes: systematic review and external validation in a Dutch primary care setting. Diabetologia 63, 1110–1119 (2020). https://doi.org/10.1007/s00125-020-05134-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-020-05134-3