Abstract
Aims/hypothesis
Our aim was to assess regression of albuminuria and its clinical consequences in type 1 diabetes.
Methods
The analysis included 3642 participants from the Finnish Diabetic Nephropathy (FinnDiane) Study with a 24 h urine sample and a history of albuminuria available at baseline. A total of 2729 individuals had normal AER, 438 a history of microalbuminuria and 475 a history of macroalbuminuria. Regression was defined as a change from a higher category of albuminuria pre-baseline to a lower category in two out of the three most recent urine samples at baseline. The impact of regression on cardiovascular events (myocardial infarction, stroke, coronary procedure) and mortality was analysed over a follow-up of 14.0 years (interquartile range 11.9–15.9).
Results
In total, 102 (23.3%) individuals with prior microalbuminuria and 111 (23.4%) with prior macroalbuminuria had regressed at baseline. For individuals with normal AER as a reference, the age-adjusted HRs (95% CI) for cardiovascular events were 1.42 (0.75, 2.68) in individuals with regression from microalbuminuria, 2.62 (1.95, 3.54) in individuals with sustained microalbuminuria, 3.15 (2.02, 4.92) in individuals with regression from macroalbuminuria and 5.49 (4.31, 7.00) in individuals with sustained macroalbuminuria. Furthermore, for all-cause and cardiovascular mortality rates, HRs in regressed individuals were comparable with those with sustained renal status at the achieved level (i.e. those who did not regress but remained at the most advanced level of albuminuria noted pre-baseline).
Conclusions/interpretation
Progression of diabetic nephropathy confers an increased risk for cardiovascular disease and premature death. Notably, regression reduces the risk to the same level as for those who did not progress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Despite vast advances in therapy, diabetic nephropathy remains a leading cause of illness and death in every third individual with type 1 diabetes. Early identification of individuals with increased risk of renal complications is therefore crucial. Microalbuminuria, present at the first stages of diabetic nephropathy, has served this purpose since the 1980s, when it emerged with an 80% progression rate to overt nephropathy [1,2,3]. However, this view has since been challenged by a number of studies suggesting much lower progression rates. Some studies even indicate that microalbuminuria regresses to normoalbuminuria more frequently than it persists or progresses [4,5,6]. Yet, in spite of the discrepancy regarding its prognostic value, no other clinical marker has so far been measured as broadly as AER in order to assess renal risk in individuals with diabetes.
In addition to predicting renal outcomes, albuminuria is also a strong predictor of cardiovascular disease [7,8,9]. Individuals with type 1 diabetes and nephropathy carry a tenfold greater risk of cardiovascular events compared with individuals with no nephropathy [10]. Small elevations of the AER translate into an increased cardiovascular risk [11]. Notably, a positive correlation between a reduction in albuminuria and cardiovascular events has been shown regardless of the baseline AER [8]. Albuminuria is further associated with premature death [12,13,14,15], which in individuals with type 1 diabetes is mainly due to cardiovascular factors [15]. Similarly, mortality rate has been reported to increase concomitantly with increasing levels of AER [14].
Data on the clinical consequences of regression of albuminuria in type 1 diabetes are scarce. Therefore, the present study was designed to assess regression of albuminuria and to study the impact of regression on cardiovascular risk and all-cause and cardiovascular mortality rates in a well-characterised cohort of individuals with type 1 diabetes.
Methods
This study is part of the Finnish Diabetic Nephropathy (FinnDiane) Study, which is a nationwide, multicentre study designed to reveal the risk factors and mechanisms of diabetic complications in individuals with type 1 diabetes, with a specific focus on nephropathy. A detailed description of the study setting has been reported previously [16]. In brief, the study includes adults (≥18 years) from over 80 participating centres (see electronic supplementary material [ESM] for a list of the FinnDiane Study centres). Baseline visits for the participants included in the current analyses were completed between 1997 and 2011. At baseline, all participants underwent a thorough clinical examination and completed standardised questionnaires regarding health and medical history in collaboration with their attending physician. Early morning blood samples were collected for the analysis of HbA1c, lipids and creatinine. eGFR was calculated with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula [17]. BP was measured twice in the sitting position after a 10 min rest, and the mean values for both systolic and diastolic BP were used in the analyses. Antihypertensive medication and specific type of agent in use was recorded.
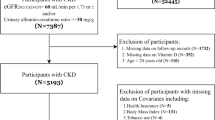
Of the original FinnDiane Study dataset comprising 5272 participants (by December 2016), 3642 met the entrance criteria for the present study. All individuals with end-stage renal disease (ESRD) were excluded, and to ensure correct diagnosis of type 1 diabetes, age at onset ≤40 years and transition to permanent insulin treatment within 1 year of diagnosis were required. The research plan was approved by the local ethics committees and the study was performed according to the Declaration of Helsinki. All participants provided written informed consent.
Measurements of urinary AER and definition of regression
Microalbuminuria was defined as a urinary AER of 30–300 mg/24 h or 20–200 μg/min, or a urinary albumin to creatinine ratio (ACR) of 2.5–25 mg/mmol for men and 3.5–35 mg/mmol for women. Normal AER was defined as the range below the lower reference value for microalbuminuria and the range above the upper reference limit for macroalbuminuria. ESRD was defined as ongoing renal replacement therapy (dialysis or renal transplantation). Figure 1 illustrates a schematic algorithm of the characterisation of the main outcome of the study. At baseline, a centrally measured 24 h urine sample, as well as the prior history of albuminuria from medical records was available. The records included either AER measurements made from 24 h urine samples or from timed overnight urine samples, or ACR measurements, depending on sample collection method at each centre. The conventionally used clinical classification of albuminuria, based on a minimum of two out of three consecutive urine samples, was accomplished for each participant pre-baseline (i.e. prior to the first FinnDiane Study visit). Every urine albumin measurement preceding the visit was screened and the most advanced stage of albuminuria was noted. Potentially confounding factors were carefully scrutinised by FinnDiane Study physicians and no urine samples collected during fever, urinary tract infections, menstruation, pregnancy or after heavy exercise were taken into account. In addition, we performed a revised classification, based on the three most recent collections at baseline, independently of the initial classification of albuminuria. It is of note that no generally accepted definition of regression of albuminuria exists. In this study we defined regression as a reversion from a higher pre-baseline status to a lower category of albuminuria measured at baseline (i.e. from microalbuminuria to normal AER, or from macro to microalbuminuria or normal AER).
Follow-up data and ascertainment of outcomes
The FinnDiane Study is an ongoing study and regular follow-up visits have been conducted since 2004. In addition to data collected during these follow-up visits, we reviewed all data available from the medical files and the laboratory databases in order to catch comprehensive follow-up information. Data collected until 31 December 2014 were used in this study and the median follow-up time was 14.0 years (interquartile range [IQR] 11.9–15.9). Follow-up data on cardiovascular events were obtained from the National Care Register of Health Care, which is the national hospital discharge register in Finland, and Statistics Finland. The combined cardiovascular outcome included first acute myocardial infarction (ICD-8/9: 410–412, ICD-10: I21–I23; www.who.int/classifications/icd/en/), cerebrovascular accident (ICD-8/9: 430–434, ICD-10: I60–I64) and coronary procedure (bypass grafting surgery or angioplasty based on the Nordic Classifications of Surgical Procedures). In the analyses concerning cardiovascular disease, only the 3449 individuals without a verified cardiovascular event at baseline were included. The individuals included were younger, had a shorter duration of diabetes, lower levels of BP and were more often women than those who were excluded (data not shown). The data on deaths were obtained from Statistics Finland. Mortality rate analyses were performed for both all-cause and cardiovascular mortality rates. The cause of death was classified as cardiovascular if the immediate, underlying or intermediate cause of death (or several of these) was recorded as disease of the circulatory system.
Statistical analyses
Descriptive clinical characteristics are presented as means ± SD (continuous variables with symmetric distributions), median with IQR (continuous variables with skewed distributions) or percentages (categorical variables). Between-group differences in continuous variables were analysed with ANOVA, Student’s t test or Mann–Whitney U test as appropriate. Between-group differences in categorical variables were tested with Pearson’s χ2 test.
Cumulative incidence curves for cardiovascular events and mortality rates (all-cause and cardiovascular) were created with the Kaplan–Meier estimator. The association between regression of albuminuria and the cardiovascular outcome and mortality rate was estimated using Cox proportional hazards modelling. The analyses are age-adjusted and presented as HRs with 95% CIs. The five groups of albuminuria status at baseline (individuals with normal AER, individuals that regressed from microalbuminuria, individuals with sustained microalbuminuria, individuals that regressed from macroalbuminuria and individuals with sustained macroalbuminuria) were pairwise compared and all p values reported from these analyses were adjusted for age. Cumulative cardiovascular mortality rates incorporating death from other causes as a competing risk was determined using the Fine–Gray method [18].
To further estimate the association between regression of albuminuria and cardiovascular disease, Cox regression analyses were performed comparing individuals with regression of albuminuria vs individuals with sustained renal status with respect to incident cardiovascular events during follow-up. Individuals with a history of microalbuminuria (regressed and sustained) and individuals with a history of macroalbuminuria (regressed and sustained) were analysed separately, and in addition, a pooled analysis with all individuals combined is presented (HR [95% CI]), as no interaction between regression and history of microalbuminuria/macroalbuminuria was observed. Selection of covariates to the multivariable models was based on univariate associations at the level of p < 0.05. First, regression of albuminuria (yes/no) was adjusted for the static confounders sex, age and age at onset of diabetes (model 2). The second multivariable model (model 3) also included dynamic confounders. In addition to HbA1c, waist-to-hip ratio and eGFR, systolic BP was selected to represent the BP level (diastolic BP excluded to avoid multicollinearity) and non-HDL-cholesterol was selected from the lipid profile because of its well-established role in predicting cardiovascular events.
All analyses were conducted using IBM SPSS Statistics for Windows, version 22.0 (IBM, Armonk, NY, USA) and R open source software version 3.2.2 (http://www.r-project.org). A two-sided p value <0.05 was considered to be statistically significant.
Results
Of the 3642 participants in this study, 2729 (74.9%) had normal AER at baseline. A total of 438 individuals (12.0%) were initially classified as microalbuminuric and 475 (13.0%) as macroalbuminuric. Of the individuals with initial microalbuminuria, 102 (23.3%) were reclassified as normoalbuminuric based on the three most recent urinary AER measurements at the baseline visit. Similarly, 111 (23.4%) of the 475 individuals with initial macroalbuminuria had regressed. Twenty individuals had regressed from macroalbuminuria to normal AER at baseline, but because of low power, these individuals were pooled with those with regression from macro to microalbuminuria in all analyses. Table 1 presents the clinical characteristics of all participants stratified into five groups by albuminuria status at baseline. The individuals who had regressed from microalbuminuria to normal AER had lower HbA1c, total cholesterol and LDL-cholesterol at baseline compared with the individuals with no change in microalbuminuria. Compared with macroalbuminuric individuals with no change, the individuals with regression from macroalbuminuria were more often women and had more favourable BP, HbA1c, eGFR and lipid profiles.
The impact of regression of albuminuria on incident cardiovascular disease
Of the 3449 individuals without previous myocardial infarction, coronary procedure or stroke, 365 (10.6%) experienced at least one confirmed cardiovascular event during the prospective phase of the study. The Kaplan–Meier curves shown in Fig. 2 illustrate the cumulative incidences of first cardiovascular event stratified by albuminuria status at baseline. The 15 year cumulative incidences were 8.0% (6.7, 9.3) for normal AER, 13.2% (6.1, 19.7) for microalbuminuria regression, 21.5% (17.4, 25.4) for sustained microalbuminuria, 28.7% (20.3, 36.2) for macroalbuminuria regression and 40.1% (36.3, 43.6) for sustained macroalbuminuria. Age-adjusted HRs for incident cardiovascular events with individuals with normal AER as a reference are presented in Table 2. After adjustment, no difference was observed between individuals with normal AER and regression from microalbuminuria and, similarly, between sustained microalbuminuria and regression from macroalbuminuria. However, individuals with regressed macroalbuminuria differed from those with sustained macroalbuminuria (p = 0.018), and a borderline significance was also noted between individuals with regressed microalbuminuria and sustained microalbuminuria (p = 0.067).
To further assess the association between regression of albuminuria and cardiovascular events, individuals with regression were compared with those with sustained renal status using Cox proportional hazards models. These results are presented in Table 3 for individuals with a history of microalbuminuria and macroalbuminuria separately, and additionally, for all individuals combined. After adjustment for the static confounders sex, age and age at onset of diabetes (model 2), a significant association between reduction of albuminuria and reduction of cardiovascular events remained for all groups. However, after the final adjustment for dynamic confounders, no independent association between regression and the adverse outcome was observed.
The impact of regression of albuminuria on mortality rate
In total, 370 individuals (10.2%) died by the end of 2014. All-cause mortality rate, stratified by albuminuria status at baseline, is presented in Fig. 3. The obtained 15 year cumulative incidences for all-cause mortality rate were 6.1% (5.2, 7.0) for normal AER, 8.8% (3.3, 14.0) for microalbuminuria regression, 19.2% (15.5, 22.8) for sustained microalbuminuria, 25.1% (18.5, 31.2) for macroalbuminuria regression and 38.1% (34.9, 41.2) for sustained macroalbuminuria. The age-adjusted HRs for all-cause mortality rate are presented in Table 2. As with cardiovascular events, the individuals who regressed showed a lower risk of death compared with those with no improvement in their renal status (p = 0.039 for regression from microalbuminuria vs sustained microalbuminuria and p = 0.003 for regression from macroalbuminuria vs sustained macroalbuminuria). However, when comparing the individuals with normal AER with the individuals whose microalbuminuria regressed, the difference was non-significant. Not unexpectedly, this was also the case for sustained microalbuminuria vs regression from macroalbuminuria.
A total of 59% (n = 199) of the causes of death were cardiovascular, and the majority of these were ischaemic heart diseases (I20–I25). The 15 year cumulative incidences for cardiovascular mortality rate are illustrated in Fig. 4 and were calculated to be 2.9% (2.1, 3.7) for normoalbuminuria, 4.7% (0.1, 9.0) for microalbuminuria regression, 9.9% (6.8, 12.9) for sustained microalbuminuria, 14.3% (8.0, 20.1) for macroalbuminuria regression and 24.1% (20.6, 27.4) for sustained macroalbuminuria. Age-adjusted HRs for cardiovascular mortality rate are presented in Table 2. Among the individuals with a history of macroalbuminuria, an association between regression of albuminuria and lower cardiovascular mortality rate was observed in the same manner as for cardiovascular events and all-cause mortality rate (p = 0.004). Individuals with normal AER vs those with microalbuminuria regression and individuals with sustained microalbuminuria vs individuals with macroalbuminuria regression were no different with regard to the outcome. Similarly, the difference between regressed and sustained microalbuminuria regarding this outcome was non-significant; however, only four individuals with microalbuminuria regression had died from cardiovascular causes during follow-up. The finding remained with the Fine–Gray competing-risk analysis, incorporating death from other causes as a competing risk, as the difference between individuals with and without regression among those with a history of macroalbuminuria reached statistical significance (p = 0.005) but the other comparisons did not (ESM Fig. 1).
Discussion
In this multicentre study of 3642 Finnish individuals with type 1 diabetes, we compared the revised classification of albuminuria, based on the three most recent urine samples at baseline, with the most advanced stage of albuminuria noted pre-baseline. Regression was defined as a reversion from a higher to a lower category of albuminuria. Consequently, 23% of individuals with pre-baseline micro and macroalbuminuria were reclassified to a less advanced stage of albuminuria at baseline, and were therefore considered to have regressed.
It has previously been shown that baseline levels of albuminuria show a positive correlation with future cardiovascular events in individuals with diabetes [7,8,9]. We not only validated these previous results but we extended them to show that regression of albuminuria in individuals with type 1 diabetes is associated with reduced risk for cardiovascular events to the same level as in those who did not progress in the first place. Interestingly, similar results have been shown in large clinical studies such as the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) Study [19], which studied hypertensive individuals with and without diabetes. The cardiovascular consequences of a 50% reduction in microalbuminuria has also been studied in individuals with type 2 diabetes [20], and an adjusted risk of 0.41 after regression was reported for these individuals. Yet, in a recent paper from DCCT/EDIC [21], remission of albuminuria was not found to reduce the cardiovascular risk in individuals with type 1 diabetes. However, there are profound differences between DCCT/EDIC and the FinnDiane Study that might explain the contradictory findings. The DCCT/EDIC represents an intervention study comparing intensive with conventional insulin therapy (DCCT) with a subsequent open-label follow-up from the close-out of the DCCT up to 30 years from baseline (EDIC). The FinnDiane Study is not an intervention but includes a substantial number of evenly distributed individuals with type 1 diabetes in Finland. Therefore, our novel observations regarding the beneficial associations between regression of albuminuria and the risk for cardiovascular events may be seen to apply to the type 1 diabetes community in general. However, the single time point ascertainment of the outcome in our study is a limitation compared with the regression assessments based on time-updated measures performed in the DCCT/EDIC. Indeed, these methodological differences could partly explain the contradictory observations made in these two studies.
Several studies are in agreement with our findings that albuminuria predicts mortality rate [12,13,14,15] and that the risk of premature death (both cardiovascular and non-cardiovascular death) increases concomitantly with declining kidney function [14]. We further extended our findings to include the impact of regression on mortality rate, and as with cardiovascular events, regression was associated with a significantly lower risk of premature death. The major cause of death in our cohort was cardiovascular disease and, as expected, cardiovascular mortality rates were reduced by regression of albuminuria in the same manner as all-cause mortality rates.
In contrast to results from the 1980s [1,2,3], some recent studies have indicated that microalbuminuria more frequently reverts to the range of normal AER than remains stable or progresses to ESRD. Two studies, one in type 1 diabetes [4] and one in type 2 diabetes [5], that defined regression of microalbuminuria as a 50% reduction in AER obtained similar results (58% and 51% 6 year cumulative incidence for regression, respectively). In the EURODIAB Prospective Complication Study [6], 50.6% of individuals with type 1 diabetes and microalbuminuria reverted to the normal AER range over a 7 year follow-up. Also in that study, regression occurred more frequently than progression, as the observed progression rate was only 13.9%.
Along similar lines, our study also proposes that microalbuminuria does not always predict inevitable progression to macroalbuminuria and ESRD. Notably, regression was more common in the aforementioned studies than our findings suggest. There may be a few explanations for this discrepancy. First, we did not have the possibility to examine changes in AER or ACR as continuous variables and theoretically we could therefore have lost valuable information by looking at only categorical variables. Yet, both Perkins et al [4] and Araki et al [5] showed surprisingly similar results in their studies by using categorical traits (regression from microalbuminuria yes/no; 59% and 54%, respectively) compared with a reduction of 50% in continuous AER measurements. Information on the overlap between the two approaches was unfortunately not shown. Our findings represent a snapshot in time not a cumulative incidence, which also may explain the lower regression rate. We cannot exclude the possibility that undetected regressions may have occurred between the initial and the revised classifications. Specifically, the scenario of fluctuating AER levels in the early stages of diabetic nephropathy, marking a dynamic rather than persistent renal injury, may remain unobserved in our study setting. In addition, the individuals in the present study were older, had longer duration of diabetes and higher BP at baseline compared with the other studies on regression of albuminuria in type 1 diabetes, which may further contribute to the differing results.
Another interesting finding of our study is the 23.4% regression from macroalbuminuria, which is strikingly similar to the regression rate in the microalbuminuric group. However, keeping in mind that the reference limits represent artificial cut-off values, this might not be surprising after all and may be a validation of the results obtained for the individuals with a history of microalbuminuria. Although the presence of macroalbuminuria has traditionally been considered a marker of irreversible renal damage, the concept of regression from higher levels of albuminuria is not completely new [22,23,24,25].
In clinical studies, intensified interventions (hypertension and glucose control) have been shown to reduce progression and even induce regression in both type 1 and type 2 diabetes, and consequently, varying regression rates have been obtained [26,27,28,29,30]. Consistent with the findings in the studies by Perkins et al [4] and the EURODIAB study group [6], we did not find evidence that renin–angiotensin–aldosterone system (RAAS) blockade is associated with regression. Regressed vs non-regressed individuals with a history of macroalbuminuria did not differ regarding the use of RAAS inhibitors (88% vs 84%), which is no surprise as the majority of individuals at this stage are designated to antihypertensive medication with RAAS inhibitors as the first choice. However, the difference in RAAS inhibitor use between regressed and non-regressed individuals with a history of microalbuminuria could be secondary to a more favourable health profile among those who had returned to normal AER.
A key strength of this study is the large, well-characterised population. The individuals in the current study represent nearly 10% of all individuals with type 1 diabetes in Finland and although the FinnDiane Study is not by definition a population-based study, the geographical distribution of participating individuals largely follows that of the general population. However, as all clinical variables were measured at the first FinnDiane Study visit, which also was the time point of the third and final urine sample included in the revised classification of albuminuria, we could not reliably assess factors associated with regression in this study. It is possible that the AERs of a number of regressed individuals levelled to less advanced stages already before entering the FinnDiane Study, but as a result of the limitations previously discussed, the exact time point for this remain unknown. Another disadvantage of the multicentre study design was the incomplete coverage of centrally measured AERs, and as a result of which we could not analyse change in AER/ACR as a continuous variable, as mentioned before. However, selection bias is less likely in a nationwide multicentre study compared with single-centre studies, and as all samples for each participant were analysed at the same centre, this should have no impact at the individual level. An additional strength of the study was the high quality and completeness of register data in addition to follow-up visits, which resulted in 100% coverage of cardiovascular events and deaths.
In conclusion, we observed regression in 23% of initially micro/macroalbuminuric individuals at baseline. A novel finding was that regression from micro to normoalbuminuria resulted in a cardiovascular risk and mortality rate no different from individuals who remained normoalbuminuric, and a similar phenomenon was seen for the individuals who regressed from macroalbuminuria compared with individuals with no change in microalbuminuria. Our observations underline the importance of keeping the AER as close to normal levels as possible in order to improve outcomes in type 1 diabetes.
Data availability
No data are available. The ethical statement and the informed consent do not allow for free data availability.
Abbreviations
- ACR:
-
Albumin/creatinine ratio
- ESRD:
-
End-stage renal disease
- FinnDiane:
-
Finnish Diabetic Nephropathy
- IQR:
-
Interquartile range
- RAAS:
-
Renin–angiotensin–aldosterone system
References
Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H (1982) Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet 319:1430–1432
Parving HH, Oxenbøll B, Svendsen PA, Christiansen JS, Andersen AR (1982) Early detection of patients at risk of developing diabetic nephropathy. A longitudinal study of urinary albumin excretion. Acta Endocrinol 100:550–555
Mogensen CE, Christensen CK (1984) Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med 311:89–93
Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS (2003) Regression of microalbuminuria in type 1 diabetes. N Engl J Med 348:2285–2293
Araki S, Haneda M, Sugimoto T, Isono M, Isshiki K, Kashiwagi A, Koya D (2005) Factors associated with frequent remission of microalbuminuria in patients with type 2 diabetes. Diabetes 54:2983–2987
Giorgino F, Laviola L, Cavallo Perin P, Solnica B, Fuller J, Chaturvedi N (2004) Factors associated with progression to macroalbuminuria in microalbuminuric type 1 diabetic patients: the EURODIAB Prospective Complications Study. Diabetologia 47:1020–1028
Gerstein HC, Mann JF, Yi Q et al (2001) Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 286:421–426
de Zeeuw D, Remuzzi G, Parving HH et al (2004) Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 110:921–927
Ljungman S, Wikstrand J, Hartford M, Berglund G (1996) Urinary albumin excretion—a predictor of risk of cardiovascular disease. A prospective 10-year follow-up of middle-aged nondiabetic normal and hypertensive men. Am J Hypertens 9:770–778
Tuomilehto J, Borch-Johnsen K, Molarius A et al (1998) Incidence of cardiovascular disease in type 1 (insulin-dependent) diabetic subjects with and without diabetic nephropathy in Finland. Diabetologia 41:784–790
Deckert T, Yokoyama H, Mathiesen E et al (1996) Cohort study of predictive value of urinary albumin excretion for atherosclerotic vascular disease in patients with insulin dependent diabetes. BMJ 312:871–874
Borch-Johnsen K, Kreiner S (1987) Proteinuria: value as predictor of cardiovascular mortality in insulin dependent diabetes mellitus. Br Med J (Clin Res Ed) 294:1651–1654
Hillege HL, Fidler V, Diercks GF et al (2002) Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 106:1777–1782
Groop PH, Thomas MC, Moran JL et al (2009) The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 58:1651–1658
Rossing P, Hougaard P, Borch-Johnsen K, Parving HH (1996) Predictors of mortality in insulin dependent diabetes: 10 year observational follow up study. BMJ 313:779–784
Thorn LM, Forsblom C, Fagerudd J et al (2005) Metabolic syndrome in type 1 diabetes: association with diabetic nephropathy and glycemic control (the FinnDiane study). Diabetes Care 28:2019–2024
Levey AS, Stevens LA, Schmid CH et al (2009) CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612
Fine J, Gray RJ (1999) A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496–509
Ibsen H, Olsen MH, Wachtell K et al (2005) Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: losartan intervention for endpoint reduction in hypertension study. Hypertension 45:198–202
Araki S, Haneda M, Koya D et al (2007) Reduction in microalbuminuria as an integrated indicator for renal and cardiovascular risk reduction in patients with type 2 diabetes. Diabetes 56:1727–1730
de Boer IH, Gao X, Cleary PA et al (2016) Albuminuria changes and cardiovascular and renal outcomes in type 1 diabetes: the DCCT/EDIC study. Clin J Am Soc Nephrol 11:1969–1977
de Boer IH, Afkarian M, Rue TC et al (2014) Renal outcomes in patients with type 1 diabetes and macroalbuminuria. J Am Soc Nephrol 25:2342–2350
Hovind P, Rossing P, Tarnow L, Toft H, Parving J, Parving HH (2001) Remission of nephrotic-range albuminuria in type 1 diabetic patients. Diabetes Care 24:1972–1977
Herbert LA, Bain RP, Verme D et al (1994) Remission of nephrotic range proteinuria in type I diabetes. Collaborative Study Group. Kidney Int 46:1688–1693
Ellis D, Lloyd C, Becker DJ, Forrest KY, Orchard TJ (1996) The changing course of diabetic nephropathy: low-density lipoprotein cholesterol and blood pressure correlate with regression of proteinuria. Am J Kidney Dis 27:809–818
Gaede P, Tarnow L, Vedel P, Parving HH, Pedersen O (2004) Remission to normoalbuminuria during multifactorial treatment preserves kidney function in patients with type 2 diabetes and microalbuminuria. Nephrol Dial Transplant 19:2784–2788
Hovind P, Rossing P, Tarnow L, Smidt UM, Parving HH (2001) Remission and regression in the nephropathy of type 1 diabetes when blood pressure is controlled aggressively. Kidney Int 60:277–283
Parving HH, Lehnert H, Bröchner-Mortensen J et al (2001) The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 345:870–878
O’Hare P, Bilbous R, Mitchell T, O’Callaghan CJ, Viberti GC (2000) Low-dose ramipril reduces microalbuminuria in type 1 diabetic patients without hypertension: results of a randomized controlled trial. Diabetes Care 23:1823–1829
Chan JC, Ko GT, Leung DH et al (2000) Long-term effects of angiotensin-converting enzyme inhibition and metabolic control in hypertensive type 2 diabetic patients. Kidney Int 57:590–600
Acknowledgements
The authors acknowledge the important role of all FinnDiane Study physicians and nurses at each centre participating in the collection of patient data (see ESM).
Contribution statement
FJJ had the main responsibility for analysing the patient data and writing the manuscript, and contributed to acquisition of data and the design of the study. CF designed the study and contributed to acquisition of data, aspects of the manuscript and critical revision of the manuscript. VH, LMT, JW, NE, AJA and MS contributed to acquisition of data and critical revision of the manuscript. VH also contributed to data analysis. P-HG contributed to acquisition of data, critical revision of the manuscript and coordination of the study. P-HG is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This study was supported by grants from the Folkhälsan Research Foundation, the Wilhelm and Else Stockmann Foundation, the Liv och Hälsa Foundation, the Helsinki University Central Hospital Research Funds, the Finnish Diabetes Research Foundation, the Finnish Medical Society (Finska Läkaresällskapet), the Finnish Cultural Foundation, the Signe and Ane Gyllenberg Foundation, the Perklén Foundation, the Novo Nordisk Foundation, Academy of Finland (134379), Tekes, Diabetes Wellness Finland, the Kidney Foundation, and the Päivikki and Sakari Sohlberg Foundation. None of these bodies played any role in data collection, analysis or preparation of the manuscript.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
MS is an advisory board member for Medtronic in Scandinavia and has received lecture fees from Eli Lilly, Medtronic Finland, MSD, Novartis, Novo Nordisk, Roche and Sanofi. P-HG has received research grants from Eli Lilly and Roche; is an advisory board member for AbbVie, AstraZeneca, Boehringer Ingelheim, Cebix, Eli Lilly, Jansen, MSD, Novartis, NovoNordisk and Sanofi; and has received lecture fees from Boehringer Ingelheim, Eli Lilly, Elo Water, Genzyme, MSD, Novartis, Novo Nordisk and Sanofi. No other potential conflicts of interest relevant to this article were reported.
Electronic supplementary material
ESM
(PDF 161 kb)
Rights and permissions
About this article
Cite this article
Jansson, F.J., Forsblom, C., Harjutsalo, V. et al. Regression of albuminuria and its association with incident cardiovascular outcomes and mortality in type 1 diabetes: the FinnDiane Study. Diabetologia 61, 1203–1211 (2018). https://doi.org/10.1007/s00125-018-4564-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-018-4564-8