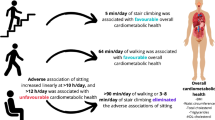
Abstract
Aims/hypothesis
Cardiovascular disease (CVD) is the most common cause of premature death and disability among patients with type 1 diabetes. Diabetic nephropathy accounts for the increased cardiovascular morbidity and mortality of these patients. We recently showed that the intensity of exercise predicts the incidence and progression of diabetic nephropathy in patients with type 1 diabetes. Little is known about the relationship between physical activity and CVD. Therefore, we studied how physical activity affects the risk of CVD events in patients with type 1 diabetes.
Methods
A 10 year follow-up study including 2180 type 1 diabetes patients from the nationwide multicentre Finnish Diabetic Nephropathy Study (FinnDiane). Leisure time physical activity (LTPA) was assessed by a previously validated self-report questionnaire. A CVD event was defined as a verified myocardial infarction, coronary procedure or stroke. Patients were analysed separately for the risk of developing a first ever CVD event and for the risk of a recurrent CVD event following a baseline event.
Results
A total of 206 patients had an incident CVD event during follow-up. A higher total LTPA and higher intensity, frequency and duration of activity were associated with a lower risk of incident CVD events. The observed association between exercise frequency and incident CVD remained significant when adjusted for classic risk factors. Exercise intensity also had a borderline effect on the recurrence-free time in patients with a major CVD event at baseline.
Conclusions/interpretation
This study suggests that exercise, particularly high frequency and high intensity exercise, may reduce the risk of CVD events in patients with type 1 diabetes.
Similar content being viewed by others
Introduction
Cardiovascular disease (CVD) is the most common cause of premature morbidity and mortality among patients with type 1 diabetes [1]. Physical activity reduces the risk of CVD in the general population and in individuals with type 2 diabetes. The beneficial effect of physical activity on cardiovascular risk factors in type 1 diabetes suggests that exercise may also reduce CVD events in these patients.
However, little is known about a potential causal relationship between physical activity and CVD in type 1 diabetes. In a cross-sectional setting, the presence of diabetic complications was associated with reduced physical activity [2, 3]. The Pittsburgh IDDM Morbidity and Mortality study demonstrated that physical activity was longitudinally associated with a reduced risk of CVD and premature mortality in male but not in female patients with type 1 diabetes [4]. On the other hand, the EURODIAB study recently demonstrated a borderline inverse association between physical activity and both all-cause mortality (for both sexes) and incident CVD (women only) in patients with type 1 diabetes [5].
Diabetic nephropathy was demonstrated to account for the increased CVD morbidity and mortality in type 1 diabetes [6]. We recently reported that the intensity of physical activity might have an impact on the incidence and progression of diabetic nephropathy [7]. In addition, an increasing body of evidence suggests that the complications of type 1 diabetes, namely CVD and diabetic nephropathy, share risk factors and develop in parallel [8].
Therefore, the aim of this study was to assess how physical activity may affect the risk of incident CVD in patients with type 1 diabetes. We prospectively investigated the relationship between baseline leisure time physical activity (LTPA) and the development of a first ever CVD event during a 10 year follow-up. We also analysed separately the relationship between physical activity and recurrence-free time from the baseline visit in a group of patients with a previous CVD event at baseline.
Methods
This paper presents the results of a longitudinal study including 2180 patients with type 1 diabetes who are participating in the ongoing nationwide, prospective, multicentre Finnish Diabetic Nephropathy Study (FinnDiane) that aims to identify clinical, biochemical, genetic and environmental risk factors for diabetic complications. Type 1 diabetes was defined as onset of diabetes before the age of 40 years and with insulin treatment initiated within 1 year of diagnosis. Patients with end-stage renal disease (ESRD) and patients with unknown renal status at baseline were excluded from this analysis (n = 245). No patients were excluded because of missing data on confounders. Altogether, 2074 patients had no major CVD events at baseline. Patients with major CVD events at baseline were analysed separately for the risk of developing an acute CVD event on top of a baseline CVD event (n = 106). The LTPA questionnaire was introduced to the FinnDiane Study at the beginning of the year 2000; consequently, baseline visits took place from 2000 to 2011. Only patients with complete data on LTPA were included.
The study protocol was approved by the ethics committees of all participating centres. Written informed consent was obtained from each patient prior to participation, and the study was performed in accordance with the Declaration of Helsinki.
LTPA questionnaire
LTPA was assessed by a self-report questionnaire. The validity and reproducibility of the questionnaire has previously been assessed and described in detail [9–11]. Patients were grouped according to their total LTPA (metabolic equivalent of task [MET]h/week) into sedentary (<10 METh/week), moderately active (10–40 METh/week) and active (>40 METh/week). The components of LTPA (intensity, frequency and duration) were also assessed. Intensity was classified as follows: (1) low intensity, with no shortness of breath and no sweating; (2) moderate intensity, with a moderate degree of shortness of breath and sweating; and (3) high intensity, with severe shortness of breath and profuse sweating. Exercise frequency was classified as <1, 1–2 or >2 sessions/week and exercise duration as ≤30, 31–60 or >60 min/session.
Clinical questionnaire
At baseline, data on medication and diabetic complications were collected via a standardised questionnaire and a thorough clinical investigation, which was completed by the attending physician. A CVD event was defined as a clinically verified myocardial infarction (ICD-8/9 410–412, ICD-10 I21–I23), coronary procedure (bypass grafting surgery or angioplasty based on the Nordic Classifications of Surgical Procedures) or either an ischaemic or haemorrhagic stroke (ICD-8/9 430–434, ICD-10 I60–I64). Follow-up events of CVD were identified from the Care Register for Health Care (HILMO) and the Finnish Causes of Death Registry and were verified from the medical records. The date of the first CVD event was defined as the date of hospital admission due to CVD. The reliability of these registries was found to be excellent for CVD [12]. Follow-up continued until the end of 2013.
Renal status
Renal status was defined based on the urinary AER in either a timed overnight or 24 h urine collection in at least two out of three consecutive measurements. Renal status was categorised as follows: normal AER, <20 μg/min or <30 mg/24 h (n = 1554); microalbuminuria, ≥20 and <200 μg/min or ≥30 and <300 mg/24 h (n = 295); or macroalbuminuria, ≥200 μg/min or ≥300 mg/24 h (n = 225). ESRD was defined as ongoing dialysis or a previous kidney transplant. BP was measured twice in the sitting position with 2 min intervals after a 10 min rest; the average of these measurements was used in the analysis. Anthropometric data were recorded and fasting blood samples were drawn for HbA1c, lipid profile and serum creatinine determinations. Smoking was assessed via a standardised questionnaire.
Statistical analyses
Data are presented as means ± SD, median and interquartile range (IQR), and percentages. ANOVA was used for normally distributed variables and the Kruskal–Wallis test was used for non-normally distributed variables. For categorical variables, the χ2 test was used. Follow-up started from the baseline visit (2000–2011) and person-years at risk were calculated until the first CVD event, death or the end of 2013. The association between different LTPA components and CVD risk was studied using Cox proportional hazard regression analyses. As the effect of LTPA and its components could be different in patients with or without diabetic nephropathy, we tested the interaction between different LTPA components and diabetic nephropathy before performing the multivariable analyses. Similarly, the interaction between LTPA and sex was tested. No component showed any significant interaction with diabetic nephropathy or sex (data not shown), suggesting that the association between LTPA or its components with incident CVD could be analysed by pooling all individuals together irrespective of kidney status or sex. In the multivariable analyses (Table 3), model 1 represents univariate analyses including only the LTPA component. In model 2, the static confounders, kidney status, sex, duration of diabetes and age at onset of diabetes were entered into the model. The final multivariable analyses (models 3–7) were conducted by adjusting for well-defined key risk factors for CVD: HbA1c, systolic BP, triacylglycerol level, smoking and BMI. The highest category for each LTPA component was used as a reference group in all analyses and the results are presented as HRs with 95% CIs. Proportionality assumption was checked by testing time–covariate interactions and plotting Schoenfeld residuals. As all proportionality assumptions held, the interaction term was not included in the models. The Kaplan–Meier method was used to estimate the cumulative incidence of CVD and the logrank test was used to test for between-group differences. A p value of <0.05 was considered statistically significant. Statistical analyses were performed using the SPSS statistical software (version 22.2. IBM, Armonk, NY, USA).
Results
Of the eligible 2074 patients without prevalent CVD events, 47.2% were men, the mean age was 38.8 ± 12.4 years, the duration of diabetes 21.7 ± 12.4 years, systolic BP 133 ± 17 mmHg, BMI 25.2 ± 3.6 kg/m2, and HbA1c 8.3 ± 1.4% (67 ± 15 mmol/mol). The median LTPA was 17.3 (95% CI 6.2, 33.4) METh. Regarding renal status, 74.9% of patients had normal AER at baseline.
Table 1 presents associations between baseline clinical characteristics and LTPA. Physical inactivity was associated with smoking, BP, sex, BMI, and triacylglycerol, HbA1c and HDL-cholesterol levels, but not with age or duration of diabetes. Electronic supplementary material (ESM) Tables 1–3 describe the relationship between intensity (ESM Table 1), frequency (ESM Table 2) and duration (ESM Table 3) of LTPA and baseline clinical variables. In general, the same baseline clinical characteristics associated with total LTPA were also associated with exercise intensity, frequency and duration.
Associations between baseline characteristics and incident CVD are summarised in ESM Table 4. During a mean follow-up of 10.3 ± 3.4 years, 206 (9.9%) patients had an incident CVD event. Patients who had a CVD event were older and heavier; had higher systolic BP, a history of smoking, a worse lipid profile and worse glycaemic control; and used antihypertensive drugs more frequently. No significant differences in sex or diastolic BP were observed.
Patients that developed a first ever CVD event during follow-up had lower total LTPA (21.9 [IQR 2.6–30.2] vs 24.3 [IQR 6.9–33.9; p = 0.042]) compared with patients who did not. Associations between the 10 year cumulative incidence of first ever CVD event and the different aspects of LTPA (total LTPA and intensity, duration and frequency of LTPA) are presented in Table 2. In general, the incidence of CVD increased with decreasing amounts of exercise irrespective of which component was studied. Total LTPA (p = 0.028), exercise intensity (p < 0.001), exercise duration (p = 0.014) and exercise frequency (p < 0.001) were all associated with incident CVD.
Table 3 presents the multivariable analyses of the effect of LTPA and intensity, duration and frequency of exercise on the risk of an incident CVD event. A higher total LTPA and longer duration, higher intensity and higher frequency of LTPA baseline were associated with a lower risk of new CVD events (model 1). Next, we added the static confounders (duration of diabetes, age at onset of diabetes, diabetic nephropathy and sex) to the multivariable analysis. The findings with respect to total LTPA and exercise intensity and frequency were unaffected by the static confounders (model 2). However, the effect of exercise duration on incident CVD events decreased to a non-significant level. In models 3–7 the confounding factors (triacylglycerol, BMI, systolic BP, HbA1c and smoking) were added sequentially; higher exercise frequency remained associated with a lower risk of CVD events in model 7. The effect of LTPA intensity was slightly reduced to become non-significant in model 6. Total LTPA was not associated with incident CVD after taking dynamic confounders into account (models 3–7). These confounding factors are themselves potentially affected by exercise. Although the interaction was not significant, we repeated this analysis in men and women separately. In men, both intensity (moderate vs high; borderline; HR 1.99 [95% CI 0.99, 3.98]) and frequency (low vs high; HR 1.95 [95% CI 1.20, 3.16]) of LTPA predicted incident CVD in the full model 7. In contrast, total LTPA (METh/week) and exercise duration seemed to be more important in women, but the effect was reduced when adjusted for the dynamic confounders (model 7). These results are presented in the ESM Tables 5 and 6.
We also analysed the relationship between LTPA and the risk of a recurrent CVD event in a small group of patients who had suffered a major CVD event before baseline (n = 106). Of these patients, 65% were men, the mean age was 53.7 ± 9.0 years, the mean duration of diabetes was 35.7 ± 10.6 years, mean BMI was 26.5 ± 4.2 kg/m2, the mean systolic BP was 145 ± 22 mmHg and mean HbA1c was 8.5 ± 1.3% (69 ± 14 mmol/mol). Fifty-six patients developed a new CVD event during follow-up. As shown in Fig. 1, the intensity of exercise had an effect on the recurrence-free time after a major CVD event at baseline (p = 0.015). The 10 year cumulative risk of a recurrent CVD event was 68.5% (95% CI, 63.4, 72.9%) in the low intensity group and 45% (95% CI 36.3, 52.5%) in the moderate intensity group, while in the high intensity group (n = 2) there were no events during follow-up. When adjusted for sex, diabetic nephropathy, age at onset of diabetes and the duration of diabetes, low vs moderate/high exercise intensity was still associated with recurrent CVD (HR 1.81 [95% CI 1.04, 3.16]). When adjusted for the other dynamic confounders (HbA1c, systolic BP, smoking, triacylglycerol and BMI), the effect decreased to a non-significant level. Total LTPA, exercise frequency and exercise duration had no effect on recurrent CVD events (data not shown).
Discussion
This prospective study showed that higher LTPA, in particular the frequency and intensity of LTPA, is associated with a decreased risk of incident CVD events in patients with type 1 diabetes. To our knowledge, this is the first time that not only the total LTPA but also its components (intensity, duration and frequency) have been explored as risk factors for incident CVD in patients with type 1 diabetes. In a separate analysis, exercise intensity also had a borderline effect on recurrence-free time in patients with a major CVD event at baseline.
We previously showed that the intensity of exercise, rather than the total amount of LTPA, predicts the progression and development of diabetic nephropathy in type 1 diabetes [7]. We have also shown that lower intensity is associated with prevalent CVD in a cross-sectional setting [2]. Therefore, the results of this study confirm and extend our previous cross-sectional and longitudinal findings. Our findings are also consistent with recent studies regarding the intensity of exercise. It is well known that long-term aerobic exercise has cardioprotective benefits. Interestingly, if the total energy expenditure of the exercise is kept constant, then high intensity exercise appears to be more cardioprotective compared with exercise of moderate intensity [13–19]. Furthermore, previous studies indicate that the intensity of LTPA is more important than the total energy expenditure in preventing hypertension and CHD and avoiding premature mortality [15, 18, 20, 21].
Based on our recent findings in diabetic nephropathy, we expected intensity to play the most important role in CVD prevention. In this study, however, exercise frequency (>2 sessions/week) appeared the strongest determinant of the risk of incident CVD. This finding is in line with the current ADA exercise recommendations for diabetes of moderate to vigorous aerobic exercise for a minimum of 150 min/week (and spreading the activity over at least 3 days during the week) or for 30 min at least 5 days a week. ADA also recommends not taking more than a 2-day break without exercise. On the other hand, the exercise recommendations for type 1 diabetes are mainly based on findings in the general population or in patients with type 2 diabetes. Thus, further studies are warranted to explore the exact mechanisms of LTPA-mediated cardioprotection and to comprehensively evaluate the potential benefits and drawbacks of high intensity or frequent exercise on CVD prevention in patients with type 1 diabetes. The mechanisms may be distinct from those responsible for the more modest benefits of classic time-consuming endurance training.
Regarding cardiovascular risk factors in patients with type 1 diabetes, exercise has been shown to improve lipid levels, insulin sensitivity and endothelial function [22]. In the general population, physical activity has been shown to improve BP, endothelial function, inflammation, sympathetic load, lipid levels, obesity, HbA1c levels and insulin sensitivity. These factors have all been implicated in the pathogenesis of diabetic complications. Therefore, we expected in the multivariable analyses that the potential confounding factors (HbA1c, systolic BP, triacylglycerol, BMI, smoking) would decrease the association with LTPA intensity and frequency to a non-significant level. Surprisingly, the association between high exercise frequency and incident CVD events was unaffected by the dynamic confounders. Therefore, our observation suggests that frequent exercise has effects in addition to those of traditional risk factors.
Previous prospective data on physical activity and CVD in type 1 diabetes are limited. In the Pittsburgh Insulin-Dependent Diabetes Mellitus Morbidity and Mortality study, an inverse association between exercise and mortality and a weak inverse association between participation in sports during high school and prevalent CVD was observed, but only in men [4]. A recent paper from the EURODIAB study found evidence of a marginally inverse association between exercise and all-cause mortality in both sexes and evidence for a borderline inverse association between exercise and incident CVD in women only [5]. In these studies, however, only the total amount of exercise was analysed, which may explain why the beneficial effects of exercise on CVD were not found in both sexes or why the association was only of borderline significance.
The strengths of this study were the large number of patients, the prospective study design and the use of a detailed physical activity questionnaire which was previously validated in the Finnish population. The questionnaire is representative (12 month LTPA correlated with maximum oxygen uptake) and has been shown to have small intraindividual variability [10]. In addition, the reliability of the hospital discharge registry, which was used to identify the CVD events, has been found to be appropriate for this purpose [12].
There were also some potential limitations with our study. Exercise was self-reported, which may not be sensitive enough. Objective measurements of exercise would be more accurate than self-report questionnaires. However, the use of devices such as accelerometers is not feasible in larger study populations such as this one and may introduce bias. On the other hand, the LTPA questionnaire has previously been used successfully in large settings [10]. Furthermore, potential changes in LTPA could not be estimated, since LTPA was only assessed at baseline. Work-related physical activity was not assessed, leading to a potential underestimation of total physical activity. Other potential confounders such as nutrition or socioeconomic status were not addressed, which may have influenced the results.
In conclusion, this is one of the few prospective studies to assess the relationship between physical activity and incident CVD in patients with type 1 diabetes. The results suggest that exercise, in particular a high frequency and high intensity exercise, may reduce the risk of CVD events. Further studies are needed to describe the mechanisms responsible for this effect and to evaluate all potential benefits and drawbacks of frequent or intensive exercise in patients with type 1 diabetes.
Abbreviations
- CVD:
-
Cardiovascular disease
- ESRD:
-
End-stage renal disease
- IQR:
-
Interquartile range
- LTPA:
-
Leisure time physical activity
References
Morrish NJ, Wang SL, Stevens LK et al (2001) Mortality and causes of death in the WHO multinational study of vascular disease in diabetes. Diabetologia 44:14–21
Wadén J, Forsblom C, Thorn LM et al (2008) Physical activity and diabetes complications in patients with type 1 diabetes: the Finnish Diabetic Nephropathy (FinnDiane) Study. Diabetes Care 31:230–232
Kriska AM, LaPorte RE, Patrick SL, Kuller LH, Orchard TJ (1991) The association of physical activity and diabetic complications in individuals with insulin-dependent diabetes mellitus: the Epidemiology of Diabetes Complications Study – VII. J Clin Epidemiol 44:1207–1214
Dorman J, Laporte R, Kuller L et al (1984) The Pittsburgh insulin-dependent diabetes mellitus (IDDM) morbidity and mortality study: mortality results. Diabetes 33:271–276
Tielemans S, Soedamah-Muthu S, De Neve M et al (2013) Association of physical activity with all-cause mortality and incident and prevalent cardiovascular disease among patients with type 1 diabetes: the EURODIAB prospective complications study. Diabetologia 56:82–91
Groop PH, Thomas MC, Moran JL et al (2009) The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 58:1651–1658
Wadén J, Tikkanen HK, Forsblom C et al (2015) Leisure-time physical activity and development and progression of diabetic nephropathy in type 1 diabetes: the FinnDiane study. Diabetologia 58:929–936
Libby P, Nathan DM, Abraham K et al (2005) Report of the national heart, lung, and blood institute-national institute of diabetes and digestive and kidney diseases working group on cardiovascular complications of type 1 diabetes mellitus. Circulation 111:3489–3493
Wadén J, Tikkanen H, Forsblom C et al (2005) Leisure time physical activity is associated with poor glycemic control in type 1 diabetic women: the FinnDiane study. Diabetes Care 28:777–782
Lakka T, Salonen J (1992) Intra-person variability of various physical activity assessments in the kuopio ischaemic heart disease risk factor study. Int J Epidemiol 21:467–472
Salonen J, Lakka T (1987) Assessment of physical activity in population studies: validity and consistency of the methods in the Kuopio ischemic heart disease risk factor study. Scand J Sports Sci 9:89–95
Sund R (2012) Quality of the Finnish hospital discharge register: a systematic review. Scand J Public Health 40:505–515
Babraj J, Vollaard N, Keast C, Guppy F, Cottrell G, Timmons J (2009) Extremely short duration high intensity interval training substantially improves insulin action in young healthy males. BMC Endocr Disord 9:3
Dunstan DW, Daly RM, Owen N et al (2002) High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care 25:1729–1736
Lee IM, Sesso HD, Oguma Y, Paffenbarger RS Jr (2003) Relative intensity of physical activity and risk of coronary heart disease. Circulation 107:1110–1116
Tjønna AE, Lee SJ, Rognmo Ø et al (2008) Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome. Circulation 118:346–354
Tremblay A, Després JP, Leblanc C et al (1990) Effect of intensity of physical activity on body fatness and fat distribution. Am J Clin Nutr 51:153–157
Paffenbarger RS Jr, Lee IM (1997) Intensity of physical activity related to incidence of hypertension and all-cause mortality: an epidemiological view. Blood Press Monit 2:115
Hu FB, Sigal RJ, Rich-Edwards JW et al (1999) Walking compared with vigorous physical activity and risk of type 2 diabetes in women. JAMA 282:1433–1439
Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB (2002) Exercise type and intensity in relation to coronary heart disease in men. JAMA 288:1994–2000
Lee IM, Hsieh C, Paffenbarger RS Jr (1995) Exercise intensity and longevity in men. JAMA 273:1179–1184
Chimen M, Kennedy A, Nirantharakumar K, Pang TT, Andrews R, Narendran P (2012) What are the health benefits of physical activity in type 1 diabetes mellitus? A literature review. Diabetologia 55:542–551
Saraheimo M, Teppo AM, Forsblom C, Fagerudd J, Groop PH (2003) Diabetic nephropathy is associated with low-grade inflammation in type 1 diabetic patients. Diabetologia 46:1402–1407
Acknowledgements
We acknowledge the important role of all the FinnDiane physicians and nurses at each participating centre for patient recruitment and the collection of samples and data, previously presented in detail [23].
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Data availability
No data are available. The ethical statement and the informed consent do not allow for free data availability.
Funding
The FinnDiane Study Group was supported by grants from the Folkhälsan Research Foundation, the Liv och Hälsa Foundation, the Wilhelm and Else Stockmann Foundation, Helsinki University Central Hospital Research Funds, the Sigrid Juselius Foundation, the Finnish Cultural Foundation, the Signe and Ane Gyllenberg Foundation, Finska Läkaresällskapet, the Academy of Finland (134379), the Novo Nordisk Foundation, and Tekes. None of these bodies played a role in data collection, analysis or preparation of the manuscript.
Duality of interest statement
P-HG has received research grants from Eli Lilly and Roche; is an advisory board member for Abbott, AbbVie, AstraZeneca, Boehringer Ingelheim, Cebix, Eli Lilly, Janssen, Medscape, MSD, Novartis and Sanofi; and has received lecture fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Genzyme, Novartis, Novo Nordisk, MSD and Sanofi. MS has received lecture fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novo Nordisk and Sanofi. All other authors declare that there is no duality of interest associated with their contribution to this manuscript.
Contribution statement
CF, JW, LMT, MR-B, MS, NE, DG, HOT and P-HG were involved in data collection; HT-D and VH analysed the data; and HT-D wrote the manuscript draft. All authors contributed to and approved the final submitted version of the manuscript. P-HG takes full responsibility for the work as a whole, including the study design, access to data and the decision to submit and publish the manuscript. P-HG is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 120 kb)
Rights and permissions
About this article
Cite this article
Tikkanen-Dolenc, H., Wadén, J., Forsblom, C. et al. Frequent and intensive physical activity reduces risk of cardiovascular events in type 1 diabetes. Diabetologia 60, 574–580 (2017). https://doi.org/10.1007/s00125-016-4189-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-016-4189-8