Abstract
Aims/hypothesis
To investigate exercise-related fuel metabolism in intermittent high-intensity (IHE) and continuous moderate intensity (CONT) exercise in individuals with type 1 diabetes mellitus.
Methods
In a prospective randomised open-label cross-over trial twelve male individuals with well-controlled type 1 diabetes underwent a 90 min iso-energetic cycling session at 50% maximal oxygen consumption (\( \overset{\cdot }{V}{\mathrm{O}}_{2 \max } \)), with (IHE) or without (CONT) interspersed 10 s sprints every 10 min without insulin adaptation. Euglycaemia was maintained using oral 13C-labelled glucose. 13C Magnetic resonance spectroscopy (MRS) served to quantify hepatocellular and intramyocellular glycogen. Measurements of glucose kinetics (stable isotopes), hormones and metabolites complemented the investigation.
Results
Glucose and insulin levels were comparable between interventions. Exogenous glucose requirements during the last 30 min of exercise were significantly lower in IHE (p = 0.02). Hepatic glucose output did not differ significantly between interventions, but glucose disposal was significantly lower in IHE (p < 0.05). There was no significant difference in glycogen consumption. Growth hormone, catecholamine and lactate levels were significantly higher in IHE (p < 0.05).
Conclusions/interpretation
IHE in individuals with type 1 diabetes without insulin adaptation reduced exogenous glucose requirements compared with CONT. The difference was not related to increased hepatic glucose output, nor to enhanced muscle glycogen utilisation, but to decreased glucose uptake. The lower glucose disposal in IHE implies a shift towards consumption of alternative substrates. These findings indicate a high flexibility of exercise-related fuel metabolism in type 1 diabetes, and point towards a novel and potentially beneficial role of IHE in these individuals.
Trial registration:
ClinicalTrials.gov NCT02068638
Funding:
Swiss National Science Foundation (grant number 320030_149321/) and R&A Scherbarth Foundation (Switzerland).
Similar content being viewed by others
Introduction
Despite its well-defined health benefits, exercise imposes high demands on individuals with type 1 diabetes [1]. Adaptation of exercise-related insulin may prevent hypoglycaemia but in clinical practice not all patients with type 1 diabetes follow treatment recommendations to adapt exercise-related insulin due to different reasons.
Intermittent high-intensity exercise (IHE) has become a widespread training modality given its well-established benefits on exercise performance and cardiovascular and metabolic variables [2, 3]. IHE consists of repeated bouts of supramaximal efforts interrupting longer periods of low to moderate intensity activity. So far, a limited number of studies have investigated IHE in type 1 diabetes, generally reporting a glycaemia-stabilising effect when compared with continuous moderate intensity exercise (CONT) [4–11]. However, exercise in the majority of these studies was of limited duration and data regarding longer IHE duration are scarce [7]. In clinical practice the problem of exercise-associated hypoglycaemia is frequently encountered during activities of longer duration, and it is currently unclear whether IHE might even be beneficial for type 1 diabetes in this context. Moreover, since the majority of previous studies investigating IHE essentially focused on blood glucose dynamics, the underlying physiological mechanisms are still ill defined. It is thought that a major glucose-stabilising effect of IHE lies in the increased hepatic glucose output stimulated by counter-regulatory hormones [12]. More recent data suggest that additional mechanisms may be involved, particularly pointing towards a change in peripheral glucose disposal induced by high-intensity exercise [13].
The aim of the present study was to investigate the metabolic and hormonal response to IHE and CONT in individuals with type 1 diabetes under conditions reflecting daily clinical practice (i.e. euglycaemia maintained by oral glucose, no specific pre-exercise insulin adaptation). The study combined stable isotope techniques, non-invasive measurement of glycogen in liver and skeletal muscle by magnetic resonance spectroscopy (MRS), and assessment of whole body substrate utilisation, and continuous glucose monitoring (CGM) to follow post-exercise glycaemia. Our main hypothesis was that the amount of exogenous glucose required to maintain euglycaemia would be lower during IHE compared with CONT. The secondary hypothesis was that such a difference would be explained by a higher consumption of glycogen during IHE.
Methods
Inclusion criteria
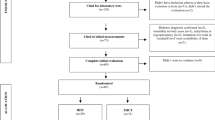
Twelve recreationally active young male adults with well-controlled, complication-free type 1 diabetes (three, multiple daily injections; nine, continuous s.c. insulin infusion) were recruited for this study. Volunteers were eligible if they had undetectable C-peptide, stable insulin regimen for at least 3 months prior to the study and were not taking any prescribed medication other than insulin. Written informed consent was obtained and the Local Ethics Committee approved the study.
Experimental design and protocol
This was a prospective, randomised, crossover trial. Randomisation was performed by sealed opaque envelopes. Preliminary study visits included an indirect calorimetry to determine basic metabolic rate (BMR), a bioimpedance analysis for lean body mass calculation (BIA 101, Akern, Pontassieve FI, Italy) and a stepwise incremental exercise test on a bicycle ergometer with breath-to-breath spiroergometry (Cardiovit AT-104 PC; Schiller, Baar, Switzerland) as described previously [14].
The exercise intervention consisted of a 90 min iso-energetic (based on energy expenditure assessed by oxygen consumption [\( \overset{\cdot }{V}{\mathrm{O}}_2 \)] measurements) cycling session at 50% maximal oxygen consumption (\( \overset{\cdot }{V}{\mathrm{O}}_{2 \max } \)) with IHE or without CONT interspersed 10 s supramaximal sprints every 10 min (at 120% of individual peak work load, followed by a 50 s recovery phase to equalise energy expenditure of the two exercise protocols, first sprint after 10 min). Interventions were separated by 2–4 weeks. Prior to the first intervention, patients were familiarised with study procedures including a full ride over 90 min with the first half involving repeated 10 s sprints. Over the entire study period glycaemia was targeted at 8 mmol/l by oral administration of a 10% dextrose solution according to a pre-specified carbohydrate (CHO) intake algorithm with individual adaptations [15]. The primary outcome was the CHO intake within the last 30 min of the exercise session.
Pre-study standardisation procedures and post-exercise follow up
During the 48 h before exercise interventions participants consumed a standardised diet with a pre-defined daily CHO quantity corresponding to 50% of their calculated energy expenditure [16] and diet was replicated prior to the second exercise intervention. Foods naturally enriched in 13C-labelled CHO were avoided to limit baseline shifts in expired 13CO2 [17]. In addition, they were requested to avoid strenuous exercise (pedometer record <5,000 steps/day), alcohol and caffeine. Patients adhered to their usual insulin regimen and glycaemia was assessed using CGM (Dexcom G4 Platinum) and frequent capillary glucose measurements. On study day participants had a standardised breakfast (1/6 of individual daily CHO amount) covered by their usual insulin dose before 07:00 hours and were admitted to the research facility at 09:30 hours. No adjustment in insulin therapy was made in anticipation of exercise and insulin dosage was the same for both trials.
After exercise patients were offered a standardised meal, fitted with a new CGM system and discharged at 17:00–18:00 hours. This was followed by an outpatient investigation of CGM, CHO intake and activity level until 06:00 hours the following day.
Sampling procedures for metabolites and hormones
On arrival an 18G cannula was inserted into an antecubital vein of each forearm. Glycaemia was measured every 10 min (YSI2300; Yellow Springs Instruments, Yellow Springs, OH, USA). Sampling for insulin, counter-regulatory hormones (catecholamines, growth hormone [GH], glucagon, cortisol) and metabolites (lactate, NEFA) was performed 50 min prior to exercise, at 10, 30, 60 and 80 min of exercise, as well as in the recovery phase 120 min after exercise. Insulin, GH and cortisol were measured using immunoassays (insulin: Architect, Abbott, Baar, Switzerland; GH:Immulite, Siemens, Zürich, Switzerland; cortisol: Modular, Roche, Rotkreuz, Switzerland). Glucagon was measured using a double radioimmunoassay (Siemens, Zürich, Switzerland) in EDTA plasma mixed with aprotinin, immediately cooled and frozen after separation. NEFA were assessed using a kit from Wako Chemicals (Dietikon, Switzerland). Lactate, pH, bicarbonate, oxygen and CO2 tension were determined electrochemically using the ABL 835/837 FLEX (Radiometer, Thalwil, Switzerland) analyser. Plasma catecholamines were quantified using ultraperformance liquid chromatography-tandem mass spectrometry (Waters Acquity UPLC/TQD, Manchester, UK) [18].
Cardiopulmonary monitoring, respiratory gas exchange and substrate oxidation
Resting CO2 consumption (\( \overset{\cdot }{V}{\mathrm{CO}}_2 \)) and \( \overset{\cdot }{V}{\mathrm{O}}_2 \) were measured immediately before exercise and in the recovery phase 120 min after exercise. The 90 min exercise session involved six spirometric recording phases, each performed over 5 min prior to the next sprint to avoid excess CO2 arising from the bicarbonate buffering system. Net substrate oxidation and energy expenditure were calculated from standard indirect calorimetry equations [19]. Heart rate was recorded continuously by a portable three channel ECG (Lifecard CF, Del Mar Reynolds Medical, Irvine, CA, USA).
Stable isotopes
Orally supplied glucose (100 g glucose/l; Glucosum monohydricum, Hänseler AG, Herisau, Switzerland) labelled with 0.5% [U-13C6]glucose was used in combination with infused [6,6-2H2]glucose (Cambridge Isotope Laboratories, Tewksbury, MA, USA). Double background enrichment measurements (blood and breath samples) were taken immediately after i.v. cannulation at test onset. Twenty minutes before exercise, a primed (0.6 mg kg−1[mmol/l glucose]−1) constant infusion of 30 μg ([6,6-2H2]glucose) kg−1 min−1 was initiated. With exercise onset the [6,6-2H2]glucose infusion rate was quadrupled to minimise changes in enrichment [20]. Measurements were obtained during the last 30 min of exercise (at 59, 69, 79 and 89 min), thereby allowing for a 60 min equilibration period, which was deemed adequate given the high glucose turnover rate during exercise. Plasma [6,6-2H2]glucose and [13C]glucose isotopic enrichment (IE) were measured using GC-MS (Hewlett-Packard Instruments, Palo Alto, CA, USA) in chemical ionisation mode as previously described [21]. 13CO2 IE was measured by isotope-ratio MS (SerCon, Crewe, UK).
Calculations of glucose kinetics
The total rate of glucose appearance (Ra) reflects the sum of hepatic glucose production (Rahep) and the rate of exogenous glucose appearing in the circulation (Raexo). Ra and rate of disappearance (Rd) during the last 30 min of exercise were calculated from [6,6-2H2]glucose dilution using Steele’s equation for non-steady state conditions assuming an effective fraction of 0.65 and a distribution volume of 0.22 l/kg [22]. Raexo was computed as plasma [13C]glucose IE/oral [13C]glucose IE × Ra. The oxidation rate of plasma glucose was calculated from 13C IE in plasma and breath, using a 13CO2 recovery factor of 1.0 [23]. The ratio of 13C-abundance in the expired air and ingested glucose drink provided an estimate of the amount of oxidised exogenous glucose. The oxidation rate of glucose released by the liver was estimated by the difference between the oxidation rate of plasma and exogenous glucose. The difference between net CHO oxidation and the oxidation of plasma glucose was used to compute oxidation of muscle glycogen. A bicarbonate correction factor assuming a bicarbonate pool of 14.2 mmol/kg was applied for estimating [13C]glucose oxidation [24].
MRS
Glycogen content in quadriceps muscle and liver was assessed using natural abundance 13C MRS [25, 26]. Measurements were performed on a 3 Tesla MR system (Trio, Siemens, Erlangen, Germany) before and after exercise. To avoid interference (e.g. increased stress response induced by the need to permanently hold an inconvenient position in the MR) a baseline MRS was scheduled 1 week prior to the experiments after an identical standardisation period. A 1H/13C-transmit–receive surface coil (RAPID Biomedical, Rimpar Germany) was tightly positioned over the tissues of interest. Positioning and repositioning of the coil were monitored by a series of localiser images and an additional fixation point on the body. 13C spectra were acquired by a pulse-and-acquire sequence using an adiabatic excitation pulse and 1H-decoupling (TR 200 ms, acquisition time 14 min [muscle] and 21 min [liver]). A chromium-doped acetone solution served as an external reference to correct for coil loading effects. In liver spatial saturation bands were applied to suppress glycogen signals (placed at carrier frequency) from the abdominal wall muscles. Glycogen content was quantified by integration of the C1 glycogen resonance at 100.5 parts per million (ppm). Glycogen levels are indicated in arbitrary units (AU).
Sample size considerations and statistical analysis
To detect a difference of 64 ml (SD 70 ml) of 20% glucose given during exercise with 5% significance and a power of 80%, a sample of ten participants was deemed necessary [7]. To account for potential dropouts the final sample size was set at 12 participants. There were no dropouts, therefore all analyses were performed with 12 individuals.
Data were analysed using Stata 13.0 (Stata Corporation, College Station, TX, USA), Matlab R2015a (The MathWorks, Natick, MA, USA) and GraphPad Prism software 5.0 (GraphPad Software, San Diego, CA, USA). Differences in hormones, metabolites and substrate oxidation were identified by paired comparison of AUCs. Glucose kinetics were compared based on values during the last 30 min of exercise. Continuous variables were analysed for normal distribution using Shapiro–Wilk test and qq-plots. Student’s paired t tests were used to identify differences for normally distributed data, and Wilcoxon’s signed rank tests were used for non-parametric data. A p value of <0.05 was considered statistically significant. Values are expressed as mean ± SEM, unless otherwise specified.
Results
Patient characteristics and pre-study standardisation
Detailed physical and functional characteristics are shown in Table 1. During the pre-study phase daily CHO intake and insulin dosage were similar (CHO: 348 ± 9 vs 359 ± 11 g, p = 0.22; insulin: 72 ± 5 vs 72 ± 4 U, p = 0.97 for IHE and CONT). The average basal insulin dose did not differ between trials (29.0 ± 2.5 vs 28.6 ± 2.5 U/24 h for IHE and CONT, p = 0.25).
Glycaemia, insulin levels and CHO requirements
During exercise, glucose (7.88 ± 0.22 vs 7.29 ± 0.20 mmol/l, p = 0.16) and insulin (150.7 ± 11.5 vs 148.5 ± 15.1 pmol/l, p = 0.50) levels did not differ between IHE and CONT. Insulin levels 120 min after end of exercise were 80.4 ± 12.2 pmol/l in IHE and 102.6 ± 20.8 pmol/l in CONT (p = 0.13). Corresponding glycaemia was 8.02 ± 0.61 and 10.09 ± 1.13 mmol/l following IHE and CONT (p = 0.08) (Fig. 1a, d). Total energy expenditure over 90 min exercise was 3.35 ± 0.15 and 3.30 ± 0.13 MJ for IHE and CONT (p = 0.67). Average heart rate was similar in IHE and CONT (142 ± 2 vs 136 ± 4 beats/min, p = 0.28). CHO requirements during the last 30 min of exercise were significantly lower in IHE (6 ± 2 g) when compared with CONT (15 ± 4 g, p = 0.02). CHO amounts supplied during the 120 min post-exercise were comparable (IHE 10 ± 3, CONT 14 ± 3 g, p = 0.22).
Effect of 90 min of IHE (black circles) and CONT (white circles) on blood glucose (a) (p = 0.16), lactate (b) (p < 0.001), NEFA (c) (p = 0.06), insulin (d) (p = 0.50), glucagon (e) (p = 0.21), GH (f) (p = 0.02), cortisol (g) (p = 0.28), adrenaline (h), (p = 0.002) and noradrenaline (i) (p < 0.001) levels
Counter-regulatory hormones and metabolites
There were no significant baseline differences for all assessed variables (Fig. 1a–i). Mean glucagon levels during exercise were 14.9 ± 0.9 and 13.3 ± 0.6 pmol/l in IHE and CONT (p = 0.21). GH was significantly higher during IHE when compared with CONT (15.6 ± 2.0 vs 10.3 ± 1.3 ng/ml, p = 0.02). Exercise-induced increase in cortisol was comparable between interventions with mean levels of 510.8 ± 35.8 and 461.5 ± 27.3 nmol/l in IHE and CONT (p = 0.28). During exercise catecholamines were significantly higher in IHE compared with CONT (p < 0.001 for noradrenaline [norepinephrine] and 0.002 for adrenaline [epinephrine]). Hormones did not differ in the post-exercise period. During IHE lactate was significantly higher when compared with CONT (7.3 ± 0.4 vs 2.0 ± 1.2 mmol/l, p < 0.001) but not at 120 min after exercise completion. During exercise significantly lower pH was observed in IHE when compared with CONT (pH: 7.27 ± 0.01 vs 7.38 ± 0.00, p < 0.001). NEFAs during exercise were 0.3 ± 0.03 mmol/l in IHE and 0.5 ± 0.04 mmol/l in CONT (p = 0.06).
Substrate oxidation and glucose kinetics
There were no significant differences in pre-exercise or post-exercise respiratory exchange ratio (RER) values between IHE and CONT (Table 2). During exercise, CHO oxidation rate was 24.74 ± 0.77 mg kg−1 min−1 in IHE and 25.73 ± 0.74 mg kg−1 min−1 in CONT (p = 0.60). Fat oxidation tended to be higher in IHE compared with CONT (2.82 ± 0.20 vs 2.30 ± 0.19 mg kg−1 min−1, p = 0.13).
Figure 2 summarises glucose kinetics during the last 30 min of exercise. Ra was significantly lower in IHE compared with CONT (6.68 ± 0.30 vs 8.02 ± 0.46 mg kg−1 min−1, p < 0.01). This was essentially due to a significantly lower rate of Raexo in the systemic circulation in IHE when compared with CONT (Raexo: 3.06 ± 0.33 vs 4.53 ± 0.45 mg kg−1 min−1, p < 0.001). In contrast, Rahep did not differ significantly between interventions (3.63 ± 0.20 vs 3.49 ± 0.29 mg kg−1 min−1 for IHE and CONT, respectively; p = 0.69). Rd was significantly lower in IHE when compared with CONT (6.80 ± 0.36 vs 8.15 ± 0.55 mg kg−1 min−1, p < 0.01). Accordingly, metabolic clearance rate was significantly lower in IHE when compared with CONT (5.31 ± 0.34 vs 6.46 ± 0.51 ml kg−1 min−1, p = 0.02).
13C IE in expired air and plasma revealed a lower oxidation rate of exogenous glucose in IHE when compared with CONT (2.62 ± 0.28 vs 3.59 ± 0.32 mg kg−1 min−1, p < 0.01). The oxidation rate of plasma glucose was 5.81 ± 0.33 vs 6.33 ± 0.38 mg kg−1 min−1 for IHE and CONT, respectively (p = 0.12). Similarly, the rate of oxidised hepatic glucose did not differ significantly between interventions (3.20 ± 0.30 vs 2.74 ± 0.31 mg kg−1 min−1 for IHE and CONT, respectively; p = 0.29). Calculated rate of muscle glycogen oxidation was similar (17.86 ± 0.77 vs 18.89 ± 0.90 mg kg−1 min−1 for IHE and CONT, respectively; p = 0.41).
Glycogen assessment by MRS
Exercise-related relative changes in intramyocellular and hepatocellular glycogen levels are depicted in Fig. 3. The 90 min exercise session resulted in a significant decrease in glycogen levels in skeletal muscle (−59 ± 8 AU in IHE, −56 ± 7 AU in CONT, p < 0.01) and liver (−54 ± 13 AU in IHE, −41 ± 12 AU, p < 0.01). The relative change in intramyocellular glycogen levels was −42.9 ± 5.3% in IHE and −40.5 ± 4.6% in CONT (p = 0.63). Liver glycogen decreased by 38 ± 8.8% in IHE and 29.2% ± 7.5% in CONT (p = 0.36).
Post-exercise follow-up overnight
Figure 4 shows results of post-exercise overnight CGM. Mean glucose was 7.85 ± 0.72 mmol/l for IHE and 8.80 ± 0.64 mmol/l for CONT (p = 0.04). The average number of hypoglycaemic (<3.5 mmol/l) events was 1.18 ± 0.42 (IHE) and 0.58 ± 0.32 (CONT, p = 0.39). Average time spent in hypoglycaemia (IHE 20 ± 9 vs CONT 10 ± 6 min, p = 0.52) and hyperglycaemia (IHE 107 ± 40 vs CONT 106 ± 35 min, p = 0.87) was similar. In both interventions, 64% of observed hypoglycaemias were above 3 mmol/l. There was no significant difference in glucose variability measures between IHE and CONT (SD: 2.45 ± 0.33 vs 2.38 ± 0.21 mmol/l, p = 0.95; CV: 0.31 ± 0.03 vs 0.28 ± 0.03, p = 0.54; mean amplitude of glycaemic excursions: 5.13 ± 0.51 vs 5.31 ± 0.55, p = 0.63).
Post-exercise CHO intake tended to be higher in IHE (233 ± 15 g) compared with CONT (212 ± 15 g, p = 0.06). The concomitantly administered total insulin (bolus and basal insulin) was comparable between the conditions (43 ± 3 vs 44 ± 2 U for IHE and CONT, respectively; p = 0.56).
Discussion
This study comprehensively assessed the hormonal and metabolic response to an IHE and CONT of 90 min in male adults with well-controlled type 1 diabetes under euglycaemia and without insulin adaptation. The principal findings were: (1) a lower amount of exogenous CHO was required to maintain euglycaemia during IHE compared with CONT; (2) this finding was not related to an increased hepatic glucose output but to a significantly lower glucose disposal in IHE compared with CONT; (3) metabolically, this was paralleled by increased levels of counter-regulatory hormones (mainly GH and catecholamines), as well as substantially higher levels of lactate in IHE; and (4) there was no measurable difference in the consumption of intramyocellular and hepatic glycogen between IHE and CONT.
The finding of ∼50% lower amounts of exogenous glucose required to uphold euglycaemia during the last 30 min of IHE confirmed our study hypothesis. However, the amount may appear disproportionate compared with the significantly smaller difference in the rates of exogenous glucose appearing in the systemic circulation (Raexo). This discrepancy may be explained by the physiological delay between glucose ingestion and systemic appearance given that Raexo in the present study was assessed during the last 30 min of exercise, exclusively.
The lower glucose disposal observed in IHE was somehow unexpected and in contrast to the secondary hypothesis. As suggested before by others, we had expected IHE, due to an increase in counter-regulatory hormones, to translate into an increased hepatic glucose output as a result of hepatic glycogenolyis and/or gluconeogenesis [12]. Indeed, we observed an increase in stress hormones, but resulting in a decreased glucose clearance instead of increased hepatic glucose output. Interestingly, it has recently been shown that a single 10 s supramaximal sprint may acutely reduce the Rd while leaving Ra unaffected in both healthy individuals and patients with type 1 diabetes [13]. This points towards a rapid change in peripheral insulin sensitivity associated with intensive exercise bouts. As shown in previous reports in individuals with type 1 diabetes [6, 8, 9], IHE in the present study induced a strong and persistent increase in counter-regulatory hormones, mainly catecholamines and GH. Previous research consistently suggests that high levels of GH are associated with a comparably rapid reduction of peripheral glucose uptake [27]. In addition, there is evidence that increased levels of catecholamines may also antagonise insulin-mediated glucose uptake in skeletal muscle [28, 29].
Another potential explanation for the lower Rd in IHE may be related to competing substrates being used by the working muscle during IHE. The significantly higher lactate levels in IHE in the present study may be interpreted in this sense. The role of lactate as a glucose-competing agent is supported by recent clamp studies showing that increased lactate utilisation was associated with decreased glucose disappearance during moderate intensity exercise [30]. Similarly, animal studies observed reduced glucose uptake by the muscle in the presence of hyperlactataemia [31–33]. Due to the design of the present study we can only speculate on potential mechanisms related to elevated lactate levels during IHE. It may stem from working muscle producing more lactate during intensive exercise bouts in IHE than in CONT. However, the finding of similar consumption of intramyocellular glycogen in IHE and CONT somewhat speaks against this being the sole explanation. Interestingly, previous research indicates that lactate may also be produced by non-working muscle or splanchnic organs [34–36]. One may thus speculate that part of lactate production during IHE may have occurred in the liver, for instance as a result of an increased hepatic glycolytic flux during IHE in individuals with type 1 diabetes in whom supraphysiological levels of insulin may have inhibited glucose-6-phosphatase [37].
Could an increased lipid oxidation account for the difference in glucose disposal between IHE and CONT? Indeed, fat oxidation was not significantly different when expressed in absolute values, but fat contribution to total energy production represented 25 ± 2% vs 21 ± 2% for fat oxidation in IHE and CONT, respectively (p = 0.03, data not shown). Of note, levels of NEFA did not significantly differ but tended to be higher during CONT. This paradox may indicate that fat oxidation relied predominantly on intramyocellular lipids (IMCL) and less on peripheral adipose tissue. Contrary to peripheral lipolysis, intramuscular degradation of lipids is not suppressed by insulin [38]. Furthermore, the greater catecholamine response in IHE may have triggered IMCL utilisation due to a stimulatory effect on hormone sensitive lipase [39]. Increased alpha-adrenergic vasoconstriction in adipose tissue during intense exercise may have further decreased circulating NEFAs by a capturing effect [28, 40]. This goes well along with the finding of comparable NEFA levels in the recovery phase.
Previous studies have raised concerns regarding an increased risk of delayed exercise-related hypoglycaemia in IHE due a depletion of glycogen stores [11]. Our findings do not point towards such an effect but we clearly acknowledge that the present study was not powered to assess this specific research question. Glycogen consumption in the present study was comparable in IHE and CONT both in the muscle and the liver. As supported by previous studies [41, 42], muscle glycogen was the most relevant fuel source in both exercise conditions (>50% of total energy yield, data not shown), independently of the prevailing insulin levels.
The strengths of the present study are its standardised and randomised design, comprehensively combining independent techniques to investigate exercise-related fuel metabolism in a setting reflecting a common clinical situation (e.g. exercise without the usually recommended adaptation of insulin doses). We, nevertheless, acknowledge several limitations. First, the complex study design limited the number of participants. Second, analysis of glucose kinetics during exercise was restricted to the last 30 min of exercise in order to allow sampling during a plateau phase of IEs. Third, oxygen consumption was not continuously measured over the entire cycling session, thereby limiting estimation of energy expenditure during and immediately after sprinting. Still, results of continuous heart rate monitoring, which is an accurate estimate for exercise-related energy expenditure, suggest that the investigated exercise protocols were entirely comparable [43]. Fourth, baseline MRS examinations were measured once for IHE and CONT on a separate day since it has been observed during the pilot phase that an MRS directly prior to exercise was too demanding and increased stress hormones. This design might have increased the variations of baseline glycogen values. Finally, we cannot fully exclude the possibility that the 13C enrichment of the oral glucose solution may have led to a small increase of the glycogen signal due to higher enrichment. However, due to the low enrichment used in the present study it can be estimated that a potential effect would be in the order of 1% or below in liver and muscle, and that an effect would be comparable in IHE and CONT, thereby not affecting direct comparison.
In conclusion, the results of the present study suggest that IHE performed over an extended time period in individuals with type 1 diabetes without prior insulin adaptation translates into lower exogenous glucose requirements compared with iso-energetic CONT exercise. Interestingly, this difference was not related to an increased consumption of hepatic or muscular glycogen in IHE, but was accompanied by a similar hepatic glucose output in both interventions. However, IHE induced a significantly decreased glucose disposal compared with CONT, implying a shift towards consumption of alternative, glucose-competing substrates in IHE. These findings corroborate the flexibility of exercise-related fuel metabolism in type 1 diabetes, indicating a novel and specific metabolic benefit of IHE in addition to its well-known effects on exercise performance. Further studies need to explore the related mechanisms in more depth.
Abbreviations
- AU:
-
Arbitrary units
- BMR:
-
Basic metabolic rate
- CGM:
-
Continuous glucose monitoring
- CHO:
-
Carbohydrate
- CONT:
-
Continuous moderate intensity exercise
- GH:
-
Growth hormone
- IE:
-
Isotopic enrichment
- IHE:
-
Intermittent high-intensity exercise
- IMCL:
-
Intramyocellular lipids
- MRS:
-
Magnetic resonance spectroscopy
- Ra:
-
Rate of glucose appearance
- Raexo :
-
Rate of exogenous glucose appearance
- Rahep :
-
Rate of hepatic glucose appearance
- Rd:
-
Rate of glucose disappearance
- RER:
-
Respiratory exchange ratio
- \( \overset{\cdot }{V}{\mathrm{O}}_2 \) :
-
Oxygen consumption
- \( \overset{\cdot }{V}{\mathrm{O}}_{2 \max } \) :
-
Maximal oxygen consumption
References
Sonnenberg GE, Kemmer FW, Berger M (1990) Exercise in type 1 (insulin-dependent) diabetic patients treated with continuous subcutaneous insulin infusion. Prevention of exercise induced hypoglycaemia. Diabetologia 33:696–703
Gibala MJ, Little JP, Macdonald MJ, Hawley JA (2012) Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol 590:1077–1084
Weston KS, Wisloff U, Coombes JS (2014) High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med 48:1227–1234
Bussau VA, Ferreira LD, Jones TW, Fournier PA (2006) The 10-s maximal sprint: a novel approach to counter an exercise-mediated fall in glycemia in individuals with type 1 diabetes. Diabetes Care 29:601–606
Bussau VA, Ferreira LD, Jones TW, Fournier PA (2007) A 10-s sprint performed prior to moderate-intensity exercise prevents early post-exercise fall in glycaemia in individuals with type 1 diabetes. Diabetologia 50:1815–1818
Campbell MD, West DJ, Bain SC et al (2014) Simulated games activity vs continuous running exercise: a novel comparison of the glycemic and metabolic responses in T1DM patients. Scand J Med Sci Sports 25:216–222
Dube MC, Lavoie C, Weisnagel SJ (2013) Glucose or intermittent high-intensity exercise in glargine/glulisine users with T1DM. Med Sci Sports Exerc 45:3–7
Guelfi KJ, Jones TW, Fournier PA (2005) Intermittent high-intensity exercise does not increase the risk of early postexercise hypoglycemia in individuals with type 1 diabetes. Diabetes Care 28:416–418
Guelfi KJ, Ratnam N, Smythe GA, Jones TW, Fournier PA (2007) Effect of intermittent high-intensity compared with continuous moderate exercise on glucose production and utilization in individuals with type 1 diabetes. Am J Physiol Endocrinol Metab 292:E865–E870
Iscoe KE, Riddell MC (2011) Continuous moderate-intensity exercise with or without intermittent high-intensity work: effects on acute and late glycaemia in athletes with type 1 diabetes mellitus. Diabet Med 28:824–832
Maran A, Pavan P, Bonsembiante B et al (2010) Continuous glucose monitoring reveals delayed nocturnal hypoglycemia after intermittent high-intensity exercise in nontrained patients with type 1 diabetes. Diabetes Technol Ther 12:763–768
Marliss EB, Vranic M (2002) Intense exercise has unique effects on both insulin release and its roles in glucoregulation: implications for diabetes. Diabetes 51(Suppl 1):S271–S283
Fahey AJ, Paramalingam N, Davey RJ, Davis EA, Jones TW, Fournier PA (2012) The effect of a short sprint on postexercise whole-body glucose production and utilization rates in individuals with type 1 diabetes mellitus. J Clin Endocrinol Metab 97:4193–4200
Jenni S, Oetliker C, Allemann S et al (2008) Fuel metabolism during exercise in euglycaemia and hyperglycaemia in patients with type 1 diabetes mellitus—a prospective single-blinded randomised crossover trial. Diabetologia 51:1457–1465
Riddell MC, Milliken J (2011) Preventing exercise-induced hypoglycemia in type 1 diabetes using real-time continuous glucose monitoring and a new carbohydrate intake algorithm: an observational field study. Diabetes Technol Ther 13:819–825
Gerrior S, Juan W, Basiotis P (2006) An easy approach to calculating estimated energy requirements. Prev Chronic Dis 3:A129
Lefebvre PJ (1985) From plant physiology to human metabolic investigations. Diabetologia 28:255–263
Dunand M, Gubian D, Stauffer M, Abid K, Grouzmann E (2013) High-throughput and sensitive quantitation of plasma catecholamines by ultraperformance liquid chromatography-tandem mass spectrometry using a solid phase microwell extraction plate. Anal Chem 85:3539–3544
Peronnet F, Massicotte D (1991) Table of nonprotein respiratory quotient: an update. Can J Sport Sci 16:23–29
Wolfe RR, Chinkes DL (2005) Isotope tracers in metabolic research: principles and practice of kinetic analysis, 2nd edn. Wiley, Chichester
Tounian P, Schneiter P, Henry S, Delarue J, Tappy L (1997) Effects of dexamethasone on hepatic glucose production and fructose metabolism in healthy humans. Am J Physiol 273:E315–E320
Steele R, Wall JS, De Bodo RC, Altszuler N (1956) Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol 187:15–24
Robert JJ, Koziet J, Chauvet D, Darmaun D, Desjeux JF, Young VR (1987) Use of 13C-labeled glucose for estimating glucose oxidation: some design considerations. J Appl Physiol (1985) 63:1725–1732
Schneiter P, Pasche O, Di Vetta V, Jequier E, Tappy L (1994) Noninvasive assessment of in vivo glycogen kinetics in humans: effect of increased physical activity on glycogen breakdown and synthesis. Eur J Appl Physiol Occup Physiol 69:557–563
Taylor R, Price TB, Rothman DL, Shulman RG, Shulman GI (1992) Validation of 13C NMR measurement of human skeletal muscle glycogen by direct biochemical assay of needle biopsy samples. Magn Reson Med 27:13–20
Gruetter R, Magnusson I, Rothman DL, Avison MJ, Shulman RG, Shulman GI (1994) Validation of 13C NMR measurements of liver glycogen in vivo. Magn Reson Med 31:583–588
Moller N, Jorgensen JO, Schmitz O et al (1990) Effects of a growth hormone pulse on total and forearm substrate fluxes in humans. Am J Physiol 258:E86–E91
Capaldo B, Napoli R, Di Marino L, Sacca L (1992) Epinephrine directly antagonizes insulin-mediated activation of glucose uptake and inhibition of free fatty acid release in forearm tissues. Metabolism 41:1146–1149
Lembo G, Capaldo B, Rendina V et al (1994) Acute noradrenergic activation induces insulin resistance in human skeletal muscle. Am J Physiol 266:E242–E247
Miller BF, Fattor JA, Jacobs KA et al (2002) Lactate and glucose interactions during rest and exercise in men: effect of exogenous lactate infusion. J Physiol 544:963–975
Lombardi AM, Fabris R, Bassetto F et al (1999) Hyperlactatemia reduces muscle glucose uptake and GLUT-4 mRNA while increasing (E1alpha)PDH gene expression in rat. Am J Physiol 276:E922–E929
Vettor R, Lombardi AM, Fabris R et al (1997) Lactate infusion in anesthetized rats produces insulin resistance in heart and skeletal muscles. Metabolism 46:684–690
Choi CS, Kim YB, Lee FN, Zabolotny JM, Kahn BB, Youn JH (2002) Lactate induces insulin resistance in skeletal muscle by suppressing glycolysis and impairing insulin signaling. Am J Physiol Endocrinol Metab 283:E233–E240
Ahlborg G (1985) Mechanism for glycogenolysis in nonexercising human muscle during and after exercise. Am J Physiol 248:E540–E545
Bergman BC, Wolfel EE, Butterfield GE et al (1999) Active muscle and whole body lactate kinetics after endurance training in men. J Appl Physiol (1985) 87:1684–1696
Wilson TH (1956) The role of lactic acid production in glucose absorption from the intestine. J Biol Chem 222:751–763
Renold AE, Hastings AB, Nesbett FB, Ashmore J (1955) Studies on carbohydrate metabolism in rat liver slices. IV. Biochemical sequence of events after insulin administration. J Biol Chem 213:135–146
Moberg E, Sjoberg S, Hagstrom-Toft E, Bolinder J (2002) No apparent suppression by insulin of in vivo skeletal muscle lipolysis in nonobese women. Am J Physiol Endocrinol Metab 283:E295–E301
Watt MJ, Stellingwerff T, Heigenhauser GJ, Spriet LL (2003) Effects of plasma adrenaline on hormone-sensitive lipase at rest and during moderate exercise in human skeletal muscle. J Physiol 550:325–332
Hodgetts V, Coppack SW, Frayn KN, Hockaday TD (1991) Factors controlling fat mobilization from human subcutaneous adipose tissue during exercise. J Appl Physiol (1985) 71:445–451
Chokkalingam K, Tsintzas K, Norton L, Jewell K, Macdonald IA, Mansell PI (2007) Exercise under hyperinsulinaemic conditions increases whole-body glucose disposal without affecting muscle glycogen utilisation in type 1 diabetes. Diabetologia 50:414–421
Robitaille M, Dube MC, Weisnagel SJ et al (2007) Substrate source utilization during moderate intensity exercise with glucose ingestion in type 1 diabetic patients. J Appl Physiol (1985) 103:119–124
Keytel LR, Goedecke JH, Noakes TD et al (2005) Prediction of energy expenditure from heart rate monitoring during submaximal exercise. J Sports Sci 23:289–297
Acknowledgements
The authors would like to thank all the volunteers for their participation and enthusiasm. Special thanks go to M. Fiedler and A. Leichtle from the Centre of Laboratory Medicine at the University Hospital Bern for great support in diagnostic aspects, to the study nurses involved in the study, and to E. Grouzmann from the University of Lausanne for support in the analysis of catecholamines.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by unrestricted grants from the Swiss National Science Foundation (grant number 320030_149321/1 to CSt) and R&A Scherbarth Foundation (Switzerland).
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
The following authors contributed to conception and design of the study: LB, CC, CB, LT, MW and CSt; acquisition of data: LB, TZ, TB, AD, NP, CSp, DP, KF, HL, RR, MW, LT, CB and CSt; analysis: LB, TZ, TB, CSp, NP, CC, DP, RR, LT, CB and CSt; and interpretation of data: LB, TZ, LT, MW, CB and CSt. All authors were involved in drafting the article (LB, TZ, LT, CB and CSt), or revising it critically for important intellectual content (LB, TZ, TB, AD, CSp, NP, CC, DP, KF, HL, RR, MW, LT, CB and CSt). All authors approved the final version to be published. CSt is responsible for the integrity of the work as a whole.
Rights and permissions
About this article
Cite this article
Bally, L., Zueger, T., Buehler, T. et al. Metabolic and hormonal response to intermittent high-intensity and continuous moderate intensity exercise in individuals with type 1 diabetes: a randomised crossover study. Diabetologia 59, 776–784 (2016). https://doi.org/10.1007/s00125-015-3854-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-015-3854-7