Abstract
Aims/hypothesis
The combined IVGTT–hyperinsulinaemic–euglycaemic clamp (Botnia clamp) allows the assessment of insulin secretion and sensitivity in one experiment. It remains unclear whether this clamp yields results comparable with those of the standard hyperinsulinaemic–euglycaemic clamp (SHEC) in diabetes patients. We hypothesised that the IVGTT induces responses affecting insulin sensitivity assessment.
Methods
Of 22 randomised diet- or metformin-treated patients with well-controlled type 2 diabetes, 19 randomly underwent a Botnia clamp and an SHEC, spaced by 2 weeks, in one clinical research centre in a crossover study. The main outcomes were whole-body and hepatic insulin sensitivity as measured by the clamp and [6,6-2H2]glucose. Substrate utilisation was assessed from indirect calorimetry and beta cell function from insulin dynamics during IVGTT.
Results
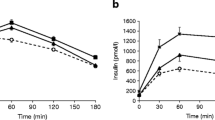
The values of whole-body insulin sensitivity obtained from Botnia clamp and SHEC were correlated (r = 0.87, p < 0.001), but also revealed intra-individual variations. Hepatic insulin sensitivity did not differ between experiments during the clamp, but differed after IVGTT. The contribution of glucose oxidation to glucose disposal increased by 2.2 ± 0.3 and 1.2 ± 0.4 mg kg fat-free mass (FFM)−1 min−1 (Botnia and SHEC, p < 0.05), whereas lipid oxidation decreased by 0.8 ± 0.1 and 0.4 ± 0.1 mg kg FFM−1 min−1 (p < 0.05) from baseline. Differences in NEFA (r = −0.60, p < 0.01), but not C-peptide (r = −0.16, p = 0.52) or hepatic insulin sensitivity between IVGTT and placebo before the clamps correlated with individual variations of insulin sensitivity.
Conclusions/interpretation
The Botnia clamp provides similar estimates of insulin sensitivity as SHEC in patients with type 2 diabetes, but changes in NEFA during IVGTT may affect insulin sensitivity and thereby the discrimination between insulin-sensitive and insulin-resistant individuals.
Trial registration: ClinicalTrials.gov NCT01397279
Funding: The study was funded by the Ministry of Science and Research of the State of North Rhine-Westphalia and the German Federal Ministry of Health, and supported in part by grants from the Federal Ministry for Research to the Centers for Diabetes Research, Helmholtz Alliance Imaging and Curing Environmental Metabolic Diseases and the Schmutzler-Stiftung.



Similar content being viewed by others
Abbreviations
- ACPR:
-
Acute C-peptide response
- AIR:
-
Acute insulin response
- BSA:
-
Body surface area
- CSI:
-
Calculated sensitivity index
- DI:
-
Disposition index
- EGP:
-
Endogenous glucose production
- FFM:
-
Fat-free mass
- GIR:
-
Glucose infusion rate
- GOX:
-
Glucose oxidation
- I:
-
Mean insulin concentration
- IGT:
-
Impaired glucose tolerance
- IL-1RA:
-
IL-1 receptor antagonist
- LOX:
-
Lipid oxidation
- M :
-
Whole-body insulin sensitivity
- MCP-1:
-
Monocyte chemoattractant protein 1
- POX:
-
Protein oxidation
- R dFFM :
-
Rate of whole-body glucose disappearance
- REE:
-
Resting energy expenditure
- SHEC:
-
Standard hyperinsulinaemic–euglycaemic clamp
References
Weyer C, Bogardus C, Mott DM, Pratley RE (1999) The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 104:787–794
Defronzo RA, Tobin JD, Andres R (1979) Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237:E214–E223
Roden M (2007) Clinical diabetes research – methods and techniques. Wiley, Chichester
Lehto M, Tuomi T, Mahtani MM et al (1997) Characterization of the MODY3 phenotype. Early-onset diabetes caused by an insulin secretion defect. J Clin Invest 99:582–591
Stancakova A, Pihlajamaki J, Kuusisto J et al (2008) Single-nucleotide polymorphism rs7754840 of CDKAL1 is associated with impaired insulin secretion in nondiabetic offspring of type 2 diabetic subjects and in a large sample of men with normal glucose tolerance. J Clin Endocrinol Metab 93:1924–1930
Tripathy D, Wessman Y, Gullstrom M, Tuomi T, Groop L (2003) Importance of obtaining independent measures of insulin secretion and insulin sensitivity during the same test: results with the Botnia clamp. Diabetes Care 26:1395–1401
Perseghin G, Ghosh S, Gerow K, Shulman GI (1997) Metabolic defects in lean nondiabetic offspring of NIDDM parents: a cross-sectional study. Diabetes 46:1001–1009
Mari A, Tura A, Natali A et al (2011) Influence of hyperinsulinemia and insulin resistance on in vivo beta-cell function: their role in human beta-cell dysfunction. Diabetes 60:3141–3147
Defronzo RA (2004) Pathogenesis of type 2 diabetes mellitus. Med Clin N Am 88:787–835
Roden M, Price TB, Perseghin G et al (1996) Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest 97:2859–2865
Stefan N, Stumvoll M, Bogardus C, Tataranni PA (2003) Elevated plasma nonesterified fatty acids are associated with deterioration of acute insulin response in IGT but not NGT. Am J Physiol Endocrinol Metab 284:E1156–E1161
Iozzo P, Lautamaki R, Geisler F et al (2004) Non-esterified fatty acids impair insulin-mediated glucose uptake and disposition in the liver. Diabetologia 47:1149–1156
Ahren B, Thorsson O (2003) Increased insulin sensitivity is associated with reduced insulin and glucagon secretion and increased insulin clearance in man. J Clin Endocrinol Metab 88:1264–1270
Rizza RA, Mandarino LJ, Gerich JE (1982) Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor detect of insulin action. J Clin Endocrinol Metab 54:131–138
Roden M, Stingl H, Chandramouli V et al (2000) Effects of free fatty acid elevation on postabsorptive endogenous glucose production and gluconeogenesis in humans. Diabetes 49:701–707
Le RD, Zick Y (2001) Recent advances in our understanding of insulin action and insulin resistance. Diabetes Care 24:588–597
Bansal P, Wang Q (2008) Insulin as a physiological modulator of glucagon secretion. Am J Physiol Endocrinol Metab 295:E751–E761
Iranmanesh A, Lawson D, Dunn B, Veldhuis JD (2011) Glucose ingestion selectively amplifies ACTH and cortisol secretory-burst mass and enhances their joint synchrony in healthy men. J Clin Endocrinol Metab 96:2882–2888
Esposito K, Nappo F, Marfella R et al (2002) Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 106:2067–2072
Ceriello A, Assaloni R, Da RR et al (2005) Effect of atorvastatin and irbesartan, alone and in combination, on postprandial endothelial dysfunction, oxidative stress, and inflammation in type 2 diabetic patients. Circulation 111:2518–2524
Kempf K, Rose B, Herder C, Kleophas U, Martin S, Kolb H (2006) Inflammation in metabolic syndrome and type 2 diabetes: impact of dietary glucose. Ann N Y Acad Sci 1084:30–48
Hotamisligil GS (1999) The role of TNFalpha and TNF receptors in obesity and insulin resistance. J Intern Med 245:621–625
Herder C, Brunner EJ, Rathmann W et al (2009) Elevated levels of the anti-inflammatory interleukin-1 receptor antagonist precede the onset of type 2 diabetes: the Whitehall II study. Diabetes Care 32:421–423
Sartipy P, Loskutoff DJ (2003) Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci U S A 100:7265–7270
Shoelson SE, Herrero L, Naaz A (2007) Obesity, inflammation, and insulin resistance. Gastroenterology 132:2169–2180
Anonymous (2012) Standards of medical care in diabetes--2012. Diabetes Care 35(Suppl 1):S11–S63
Schmid AI, Szendroedi J, Chmelik M, Krssak M, Moser E, Roden M (2011) Liver ATP synthesis is lower and relates to insulin sensitivity in patients with type 2 diabetes. Diabetes Care 34:448–453
Segal KR, Van LM, Fitzgerald PI, Hodgdon JA, Van Itallie TB (1988) Lean body mass estimation by bioelectrical impedance analysis: a four-site cross-validation study. Am J Clin Nutr 47:7–14
Szendroedi J, Anderwald C, Krssak M et al (2009) Effects of high-dose simvastatin therapy on glucose metabolism and ectopic lipid deposition in nonobese type 2 diabetic patients. Diabetes Care 32:209–214
Weickert MO, Loeffelholz CV, Roden M et al (2007) A Thr94Ala mutation in human liver fatty acid-binding protein contributes to reduced hepatic glycogenolysis and blunted elevation of plasma glucose levels in lipid-exposed subjects. Am J Physiol Endocrinol Metab 293:E1078–E1084
Schadewaldt P, Nowotny B, Strassburger K, Kotzka J, Roden M (2013) Indirect calorimetry in humans: a postcalorimetric evaluation procedure for correction of metabolic monitor variability. Am J Clin Nutr 97:763–773
Frayn KN (1983) Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 55:628–634
Nowotny B, Nowotny PJ, Strassburger K, Roden M (2012) Precision and accuracy of blood glucose measurements using three different instruments. Diabet Med 29:260–265
Herder C, Schneitler S, Rathmann W et al (2007) Low-grade inflammation, obesity, and insulin resistance in adolescents. J Clin Endocrinol Metab 92:4569–4574
Krebs M, Krssak M, Nowotny P et al (2001) Free fatty acids inhibit the glucose-stimulated increase of intramuscular glucose-6-phosphate concentration in humans. J Clin Endocrinol Metab 86:2153–2160
Tura A, Sbrignadello S, Succurro E, Groop L, Sesti G, Pacini G (2010) An empirical index of insulin sensitivity from short IVGTT: validation against the minimal model and glucose clamp indices in patients with different clinical characteristics. Diabetologia 53:144–152
Nowotny B, Zahiragic L, Krog D et al (2013) Mechanisms underlying the onset of oral lipid-induced skeletal muscle insulin resistance in humans. Diabetes 62:2240–2248
Mari A, Stojanovska L, Proietto J, Thorburn AW (2003) A circulatory model for calculating non-steady-state glucose fluxes. Validation and comparison with compartmental models. Comput Methods Prog Biomed 71:269–281
Morbiducci U, Di BG, Kautzky-Willer A, Deriu MA, Pacini G, Tura A (2011) Identification of a model of non-esterified fatty acids dynamics through genetic algorithms: the case of women with a history of gestational diabetes. Comput Biol Med 41:146–153
Tam CS, Xie W, Johnson WD, Cefalu WT, Redman LM, Ravussin E (2012) Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes Care 35:1605–1610
Bush NC, Basu R, Rizza RA, Nair KS, Khosla S, Jensen MD (2012) Insulin-mediated FFA suppression is associated with triglyceridemia and insulin sensitivity independent of adiposity. J Clin Endocrinol Metab 97:4130–4138
Szendroedi J, Schmid AI, Chmelik M et al (2007) Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. PLoS Med 4:e154
Samuel VT, Petersen KF, Shulman GI (2010) Lipid-induced insulin resistance: unravelling the mechanism. Lancet 375:2267–2277
Jornayvaz FR, Shulman GI (2012) Diacylglycerol activation of protein kinase Cepsilon and hepatic insulin resistance. Cell Metab 15:574–584
Bikman BT, Summers SA (2011) Ceramides as modulators of cellular and whole-body metabolism. J Clin Invest 121:4222–4230
Santomauro AT, Boden G, Silva ME et al (1999) Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes 48:1836–1841
Galgani JE, Moro C, Ravussin E (2008) Metabolic flexibility and insulin resistance. Am J Physiol Endocrinol Metab 295:E1009–E1017
Roden M, Perseghin G, Petersen KF et al (1996) The roles of insulin and glucagon in the regulation of hepatic glycogen synthesis and turnover in humans. J Clin Invest 97:642–648
Moore MC, Satake S, Baranowski B, Hsieh PS, Neal DW, Cherrington AD (2002) Effect of hepatic denervation on peripheral insulin sensitivity in conscious dogs. Am J Physiol Endocrinol Metab 282:E286–E296
Tonelli J, Kishore P, Lee DE, Hawkins M (2005) The regulation of glucose effectiveness: how glucose modulates its own production. Curr Opin Clin Nutr Metab Care 8:450–456
Acknowledgements
We thank F. Schwarz for her assistance in the clamp experiments and U. Partke, I. Latta, R. Schreiner, D. Scheibelhut, D. Seeger, B. Platzbecker and C. Preuß for technical assistance (all at the German Diabetes Center, Düsseldorf, Germany). We thank A. Mari (Institute of Biomedical Engineering, Padova, Italy) for the calculation of the non-steady-state EGP. Some of the data have previously been presented as an abstract at the 73rd Scientific Sessions of the American Diabetes Association in Chicago, IL, USA in 2013.
Funding
This work was supported by the Ministry of Science and Research of the State of North Rhine-Westphalia (MIWF NRW) and the German Federal Ministry of Health (BMG). This study was supported in part by grants from the Federal Ministry for Research (BMBF) to the Centers for Diabetes Research (DZD e.V.), Helmholtz Alliance Imaging and Curing Environmental Metabolic Diseases (ICEMED) and the Schmutzler-Stiftung.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
MR designed the study and headed the clinical experiments. SK, SP and BN researched the data. SK wrote the first draft of the manuscript and coordinated the inclusion of specific sections as outlined. PJN and CH conducted and wrote aspects of the laboratory analyses. KS supervised and interpreted the statistical analyses of the data. GP calculated indices of beta cell function and wrote the respective sections. All the authors contributed substantially to aspects of study design or the acquisition of data, contributed to drafting of the article or revised it critically for important intellectual content and gave final approval to the version to be published. MR is responsible for the integrity of the work as a whole.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Fig. 1
(PDF 11 kb)
ESM Fig. 2
(PDF 94 kb)
ESM Fig. 3
(PDF 88 kb)
ESM Fig. 4
(PDF 85 kb)
ESM Table 1
(PDF 81 kb)
ESM Table 2
(PDF 149 kb)
Rights and permissions
About this article
Cite this article
Kahl, S., Nowotny, B., Piepel, S. et al. Estimates of insulin sensitivity from the intravenous-glucose-modified-clamp test depend on suppression of lipolysis in type 2 diabetes: a randomised controlled trial. Diabetologia 57, 2094–2102 (2014). https://doi.org/10.1007/s00125-014-3328-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-014-3328-3