Abstract
Aims/hypothesis
Dysregulation of 11β-hydroxysteroid dehydrogenase (11β-HSD) enzyme activities are implicated in the pathogenesis of obesity and insulin resistance. The aim of the study was to determine whether hepatic 11β-HSD type 1 (11β-HSD-1) enzyme activity differs in people with and without obesity and type 2 diabetes.
Methods
We measured hepatic 11β-HSD-1 activity in the overnight fasted state in 20 lean non-diabetic participants (LND), 21 overweight/obese non-diabetic participants (OND) and 20 overweight/obese participants with type 2 diabetes (ODM) using a non-invasive approach. One mg doses of [9,12,12-2H3]cortisol (D cortisol) and [4-13C]cortisone ([13C]cortisone) were ingested, while [1,2,6,7-3H]cortisol ([3H] cortisol) was infused intravenously to enable concurrent measurements of first-pass hepatic extraction of ingested D cortisol and hepatic conversion of ingested [13C]cortisone to C13 cortisol derived from the ingested cortisone (a measure of 11β-HSD-1 activity in the liver) using an isotope dilution technique. One-way ANOVA models and Kruskal–Wallis tests were used to test the hypothesis.
Results
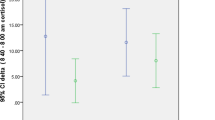
Plasma D cortisol and C13 cortisol concentrations were lower in OND than in LND (p < 0.05) over 6 h of the study. There was no difference (p = 0.15) in C13 and D cortisol concentrations between OND and ODM and between LND and ODM for the same study period. Hepatic conversion of [13C]cortisone to C13 cortisol was similar between groups.
Conclusions/interpretation
Hepatic conversion of [13C]cortisone to C13 cortisol did not differ between the groups studied. We conclude that hepatic 11β-HSD-1 activity is similar in individuals who are overweight/obese or who have type 2 diabetes.





Similar content being viewed by others
Abbreviations
- [13C]Cortisone:
-
[4-13C]Cortisone
- [3H]Cortisol:
-
[1,2,6,7-3H]Cortisol
- C13 Cortisol:
-
Cortisol derived from ingested [13C]cortisone
- CTSA:
-
Mayo Clinic Center for Translational Science Activities
- D Cortisol:
-
[9,12,12-2H3]Cortisol
- D Cortisone:
-
[9,12,12-2H3]Cortisone
- dpm:
-
Disintegrations per minute
- 11β-HSD:
-
11β-Hydroxysteroid dehydrogenase
- 11β-HSD-1:
-
11β-Hydroxysteroid dehydrogenase type 1
- LND:
-
Lean non-diabetic participants
- MPE:
-
Mole per cent enrichment
- MR:
-
Molar ratio
- OND:
-
Overweight/obese non-diabetic participants
- ODM:
-
Overweight/obese participants with type 2 diabetes
- Ra:
-
Rate of appearance
References
Walker BR (2004) Is ‘Cushing’s disease of the omentum’ an affliction of mouse and men? Diabetologia 47:767–769
Moore JS, Monson JP, Kaltsas G et al (1999) Modulation of 11β-hydroxysteroid dehydrogenase isozymes by growth hormone and insulin-like growth factor: in vivo and in vitro studies. J Clin Endocrinol Metab 84:4172–4177
Jamieson PM, Chapman KE, Edwards CRW, Seckl JR (1995) 11β-Hydroxysteroid dehydrogenase is an exclusive 11β-reductase in primary cultures of rat hepatocytes: effect of physicochemical and hormonal manipulations. Endocrinology 136:4754–4761
Paulmyer-Lacroix O, Boullu S, Oliver C, Alessi M-C, Grino M (2002) Expression of the mRNA coding for 11β-hydroxysteroid dehydrogenase type 1 in adipose tissue from obese patients: an in situ hybridization study. J Clin Endocrinol Metab 87:2701–2705
Bujalska IJ, Kumar S, Stewart PM (1997) Does central obesity reflect ‘Cushing’s disease of the omentum’? Lancet 349:1210–1213
Whorwood CB, Donovan SJ, Flanagan D, Phillips DIW, Byrne CD (2002) Increased glucocorticoid receptor expression in human skeletal muscle cells may contribute to the pathogenesis of the metabolic syndrome. Diabetes 51:1066–1075
Anagnostis P, Katsiki N, Adamidou F et al (2013) 11beta-Hydroxysteroid dehydrogenase type 1 inhibitors: novel agents for the treatment of metabolic syndrome and obesity-related disorders? Metabolism 62:21–33
Basu R, Basu A, Grudzien M et al (2009) Liver is the site of splanchnic cortisol production in obese non-diabetic humans. Diabetes 58:39–45
Stewart PM, Boulton A, Kumar S, Clark PMS, Shackleton CHL (1999) Cortisol metabolism in human obesity: impaired cortisone→cortisol conversion in subjects with central adiposity. J Clin Endocrinol Metab 84:1022–1027
Andrew R, Westerbacka J, Wahren J, Yki-Järvinen H, Walker BR (2005) The contribution of visceral adipose tissue to splanchnic cortisol production in healthy humans. Diabetes 54:1364–1370
Andrew R, Phillips DI, Walker BR (1998) Obesity and gender influence cortisol secretion and metabolism in man. J Clin Endocrinol Metab 83:1806–1809
Rask E, Olsson T, Söderberg S et al (2001) Tissue-specific dysregulation of cortisol metabolism in human obesity. J Clin Endocrinol Metab 86:1418–1421
Rask E, Walker BR, Söderberg S et al (2002) Tissue-specific changes in peripheral cortisol metabolism in obese women: increased adipose 11β-hydroxysteroid dehydrogenase type 1 activity. J Clin Endocrinol Metab 87:3330–3336
Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP (2009) The pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab 94:2692–2701
Basu R, Singh RJ, Basu A et al (2005) Obesity and type 2 diabetes do not alter splanchnic cortisol production in humans. J Clin Endocrinol Metab 90:3919–3926
Draper N, Stewart PM (2005) 11β-Hydroxysteroid dehydrogenase and the pre-receptor regulation of corticosteroid hormone action. J Endocrinol 186:251–271
Basu R, Singh RJ, Basu A et al (2004) Splanchnic cortisol production occurs in humans: evidence for conversion of cortisone to cortisol via the 11-β hydroxysteroid dehydrogenase (11-β HSD) type 1 pathway. Diabetes 53:2051–2059
Taylor RL, Machacek D, Singh RJ (2002) Validation of a high-throughput liquid chromatography–tandem mass spectrometry method for urinary cortisol and cortisone. Clin Chem 48:1511–1519
Basu A, Basu R, Shah P et al (2000) Effects of type 2 diabetes on the ability of insulin and glucose to regulate splanchnic and muscle glucose metabolism: evidence for a defect in hepatic glucokinase activity. Diabetes 49:272–283
Steele R, Wall J, DeBodo R, Altszuler N (1956) Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol 187:15–24
Basu R, Di Camillo B, Toffolo G et al (2003) Use of a novel triple-tracer approach to assess postprandial glucose metabolism. Am J Physiol Endocrinol Metab 284:E55–E69
Hughes KA, Manolopoulos KN, Iqbal J et al (2012) Recycling between cortisol and cortisone in human splanchnic, subcutaneous adipose, and skeletal muscle tissues in vivo. Diabetes 61:1357–1364
Stimson RH, Andrew R, McAvoy NC, Tripathi D, Hayes PC, Walker BR (2011) Increased whole-body and sustained liver cortisol regeneration by 11beta-hydroxysteroid dehydrogenase type 1 in obese men with type 2 diabetes provides a target for enzyme inhibition. Diabetes 60:720–725
Whorwood CB, Ricketts ML, Stewart PM (1994) Epithelial cell localization of type 2 11 beta-hydroxysteroid dehydrogenase in rat and human colon. Endocrinology 135:2533–2541
Shimojo M, Ricketts ML, Petrelli MD et al (1997) Immunodetection of 11 beta-hydroxysteroid dehydrogenase type 2 in human mineralocorticoid target tissues: evidence for nuclear localization. Endocrinology 138:1305–1311
Ricketts ML, Verhaeg JM, Bujalska I, Howie AJ, Rainey WE, Stewart PM (1998) Immunohistological localization of type 1 11β-hydroxysteroid dehydrogenase in human tissues. J Clin Endocrinol Metab 83:1325–1335
Basu R, Singh R, Basu A, Johnson CM, Rizza RA (2006) Effect of nutrient ingestion on total-body and splanchnic cortisol production in humans. Diabetes 55:667–674
Tomlinson JW, Finney J, Hughes BA, Hughes SV, Stewart PM (2008) Reduced glucocorticoid production rate, decreased 5 alpha-reductase activity, and adipose tissue insulin sensitization after weight loss. Diabetes 57:1536–1543
Acknowledgements
We give our deepest appreciation and thanks to R. A. Rizza (Mayo Clinic) for his valuable and constructive suggestions. We are deeply indebted to the research participants. Our sincere thanks go to the staff of the CTSA, the Clinical Research Unit, the CTSA Immunochemical Core Laboratory, and the CTSA Metabolomics Core Facility (M. Persson). We wish to thank Mayo Clinic staff; C. Shonkwiler (research nurse), P. Reich (research assistant), B. Dicke (laboratory technician) and M. Slama (laboratory technician) for their technical assistance; B. McConahey (laboratory technician) for technical assistance and graphic design; and C. Speltz (medical secretary) and L. Kvall Boynton (secretary) for assistance with preparation of the manuscript.
Funding
The work was supported by National Institutes of Health grants R01 DK29953 and UL1 TR000135 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
SD researched the data, managed the data handling and data analyses, and assisted with writing the manuscript. BN assisted with the acquisition of data and conduct of the study. VP assisted with the conduct of the study and the acquisition and analyses of data. REC and RKL assisted with the acquisition of data and the statistical analyses. RJS assisted with the mass spectrometry data acquisition and analyses. AB assisted with the study design, data analyses and manuscript editing. RB assisted with the study design, researched the data, assisted with the conduct of the study, data analyses and writing the manuscript. All authors were involved with editing the manuscript and approving the final version to be published. RB is the guarantor of this manuscript and is responsible for the integrity of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dube, S., Norby, B., Pattan, V. et al. Hepatic 11β-hydroxysteroid dehydrogenase type 1 activity in obesity and type 2 diabetes using a novel triple tracer cortisol technique. Diabetologia 57, 1446–1455 (2014). https://doi.org/10.1007/s00125-014-3240-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-014-3240-x