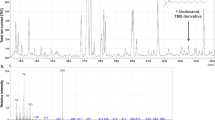
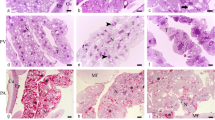
Abstract
Insects employ different defense strategies against fungal infections and chemicals. We aimed to identify the lipid compositions of the fat body of Zophobas morio larvae before and after fungal infection with the entomopathogenic fungus Metarhizium flavoviride. We used gas chromatography-mass spectrometry to analyze lipid extracts of the fat body isolated of Z. morio 2, 5, and 7 days after fungal infection (treatment group) and compared it with the lipid extracts in a control group injected with physiological isotonic saline. In all the samples, fatty acids were the most abundant compound found in the fat body extracts, with hexadecanoic acid/C16:0 being the most abundant lipid. However, the types and concentrations of lipids changed after fungal infection, likely as an immune response. The most considerable changes occurred in the concentrations of long-chain fatty acids, i.e., hexadecanoic acid/C16:0, octadecenoic acid/C18:1, and octadecanoic acid/C18:0. Contents of methyl ester increased significantly after infection, but that of other esters, especially octanoic acid decyl ester/OADE, decreased on the 5th day after infection. To the best of our knowledge, this is the first detailed analysis of the changes in the lipid composition of the fat body of Z. morio larvae as a result of fungal infection. Our results suggest that entomopathogenic fungal infection affects the internal lipid composition of insects, potentially as a way of adjusting to such infection. These results can help understand infection processes and defense strategies of insects against fungal infection. Ultimately, they can contribute to the creation of more effective chemicals against pest insects.








Similar content being viewed by others
References
Akiduki G, Imanishi S (2007) Establishment of a lipid accumulation model in an insect cell line. Arch Insect Biochem Physiol 66:109–121
Angelo IC, Gôlo PS, Perinotto WM, Camargo MG, Quinelato S, Sá FA, Pontes EG, Bittencourt VR (2013) Neutral lipid composition changes in the fat bodies of engorged females Rhipicephalus microplus ticks in response to fungal infections. Parasitol Res 112:501–509
Arrese EL, Soulages JL (2010) Insect fat body, energy, metabolism, and regulation. An Rev Entomol 55:207–225
Augustyniuk-Kram A, Kram KJ (2012) Entomopathogenic fungi as an important natural regulator of insect outbreaks in forests (Review). In Blanco JA (ed) Forest Ecosystems–More than Just Trees. InTech Rijeka, pp 265
Boguś MI, Kędra E, Bania J, Szczepanik M, Czygier M, Jabłoński P, Pasztaleniec A, Samborski J, Mazgajska J, Polanowski A (2007) Different defense strategies of Dendrolimus pini, Galleria mellonella, and Calliphora vicina against fungal infection. J Insect Physiol 53:909–922
Boguś MI, Czygier M, Gołębiowski M, Kędra E, Kucińska J, Mazgajska J, Samborski J, Wieloch W, Włóka E (2010) Effects of insect cuticular fatty acids on in vitro growth and pathogenicity of the entomopathogenic fungus Conidiobolus coronatus. Exp Parasitol 125:400–408
Bridge PD, Williams MAJ, Prior C, Paterson RRM (1993) Morphological, biochemical and molecular characteristics of Metarhizium anisopliae and M. flavoviride. J Gen Microbiol 139:1163–1169
Canavoso LE, Jouni ZE, Karnas KJ, Pennington JE, Wells MA (2001) Fat metabolism in insects. An Rev Nutrition 21:23–46
Carrillo D, Dunlap CA, Avery PB, Navarrete J, Duncan RE, Jackson MA, Behle RW, Cave RD, Crane J, Rooney AP, Peña JE (2015) Entomopathogenic fungi as biological control agents for the vector of the laurel wilt disease, the redbay ambrosia beetle, Xyleborus glabratus (Coleoptera: Curculionidae). Biol Control 81:44–50
Cerkowniak M, Puckowski A, Stepnowski P, Gołębiowski M (2013) The use of chromatographic techniques for the separation and the identification of insect lipids. J Chromatogr B 937:67–78
Cheon HM, Shin SW, Bian G, Park JH, Raikhel AS (2006) Regulation of lipid metabolism genes, lipid carrier protein lipophorin, and its receptor during immune challenge in the mosquito Aedes aegypti. J Biol Chem 281:8426–8435
Cuthbertson AGS, Walters KFA, Deppe C (2005) Compatibility of the entomopathogenic fungus Lecanicillium muscarium and insecticides for eradication of sweetpotato whitefly, Bemisia tabaci. Mycopathol 160:35–41
Ferron P (1978) Biological control of insect pests by entomogenous fungi. Annu Rev Entomol 23:409–442
Folch J, Lees M, Sloane-Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Gabarty A, Salem HM, Fouda MA, Abas AA, Ibrahim AA (2014) Pathogencity induced by the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in Agrotis ipsilon (Hufn.). J Radiat Res Appl Sci 7:95–100
Gołębiowski M, Boguś MI, Paszkiewicz M, Stepnowski P (2011) Cuticular lipids of insects as potential biofungicides: methods of lipid composition analysis. Anal Bioanal Chem 399:3177–3191
Gołębiowski M, Cerkowniak M, Dawgul M, Kamysz W, Boguś MI, Stepnowski P (2013a) The antifungal activity of the cuticular and internal fatty acid methyl esters and alcohols in Calliphora vomitoria. Parasitology 140:972–985
Gołębiowski M, Cerkowniak M, Boguś MI, Włóka E, Dawgul M, Kamysz W, Stepnowski P (2013b) Free fatty acids in the cuticular and internal lipids of Calliphora vomitoria and their antimicrobial activity. J Insect Physiol 59:416–429
Gołębiowski M, Cerkowniak M, Urbanek A, Słocińska M, Rosiński G, Stepnowski P (2014a) Adipokinetic hormone induces changes in the fat body lipid composition of the beetle Zophobas atratus. Peptides 58:65–73
Gołębiowski M, Urbanek A, Oleszczak A, Dawgul M, Kamysz W, Boguś MI, Stepnowski P (2014b) The antifungal activity of fatty acids of all stages of Sarcophaga carnaria L. (Diptera: Sarcophagidae). Microbiol Res 169:279–286
Gołębiowski M, Cerkowniak M, Urbanek A, Dawgul M, Kamysz W, Bogus MI, Stepnowski P (2015) Identification and antifungal activity of the novel organic compounds found in cuticular and internal lipids of medically important flies. Microbiol Res 170:213–222
Guo L, Blomquist GJ (1991) Identification, accumulation, and biosynthesis of the cuticular hydrocarbons of the southern armyworm, Spodoptera eridania (cramer) (lepidoptera: Noctuidae). Arch Insect Biochem Physiol 16:19–30
Gutierrez AC, Gołębiowski M, Pennisi M, Peterson G, García JJ, Manfrino RG, Lastra CCL (2015) Cuticle fatty acid composition and differential susceptibility of three species of cockroaches to the entomopathogenic fungi Metarhizium anisopliae (Ascomycota, Hypocreales). J Econ Entomol 108:752–760
Hajek AE, Delalibera I Jr (2010) Fungal pathogens as classical biological control agents against arthropods. BioControl 55:147–158
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9
Hoffmann JA (1995) Innate immunity of insects. Curr Opin Immunol 7:4–10
Hooper GHS, Brown WV, Lacey MJ, Hunter D (1996) Cuticular hydrocarbons of the Australian plague locust, Chortoicetes terminifera (Walker) (Orthoptera: Acrididae), collected from widely separated geographical locations. Aust J Entomol 35:257–262
Howard RW, Baker JE (2003) Cuticular hydrocarbons and wax esters of the ectoparasitoid Habrobracon hebetor: ontogenetic, reproductive, and nutritional effects. Arch Insect Biochem Physiol 53:1–18
Howard RW, Stanley-Samuelson DW (1996) Fatty acid composition of fat body and Malpighian tubules of the tenebrionid beetle, Zophobas atratus: significance in eicosanoid-mediated physiology. Comp Biochem Physiol B 115:429–437
Jurenka R (2004) Insect pheromone biosynthesis. In: The chemistry of pheromones and other semiochemicals I. Topics in Current Chemistry Vol. 239. Springer Berlin Heidelberg, 97–132
Kershaw MJ, Moorhouse ER, Bateman R, Reynolds SE, Charnley AK (1999) The role of destruxins in the pathogenicity of Metarhizium anisopliae for three species of insect. J Invertebr Pathol 74:213–223
Keyser CA, Licht HHDF, Steinwender BM, Meyling NV (2015) Diversity within the entomopathogenic fungal species Metarhizium flavoviride associated with agricultural crops in Denmark. BMC Microbiol 15:249
Khachatourians GG, Qazi SS (2008) Entomopathogenic fungi: biochemistry and molecular biology. In: Brackage AA (ed) The Mycota VI: human and animal relationships. Springer, Heidelberg, pp 33–61
Koštál V, Simek P (1998) Changes in fatty acid composition of phospholipids and triacylglycerols after cold-acclimation of an aestivating insect prepupa. J Comp Physiol B 168:453–460
Koštál V, Urban T, Řimnáčová L, Berková P, Šimek P (2013) Seasonal changes in minor membrane phospholipid classes, sterols and tocopherols in an overwintering insect, Pyrrhocoris apterus. J Insect Physiol 59:934–941
Kühnlein RP (2012) Lipid droplet-based storage fat metabolism in Drosophila. J Lipid Res 53:1430–1436
Lockey KH (1988) Lipids of the insect cuticle: origin, composition and function. Comp Biochem Physiol B 89:595–645
Lorenz MW, Gäde G (2009) Hormonal regulation of energy metabolism in insects as a driving force for performance. Integr Comp Biol 49:380–392
Mirth CK, Riddiford LM (2007) Size assessment and growth control: how adult size is determined in insects. BioEssays 29:344–355
Moore D, Reed M, Le Patourel G, Abraham YJ, Prior C (1992) Reduction of feeding by the desert locust, Schistocerca gregaria, after infection with Metarhizium flavoviride. J Invertebr Pathol l60:304–307
Mullen L, Goldsworthy G (2003) Changes in lipophorins are related to the activation of phenoloxidase in the haemolymph of Locusta migratoria in response to injection of immunogens. Insect Biochem Mol Biol 33:661–670
Murray ZL, Keyzers RA, Barbieri RF, Digby AP, Lester PJ (2016) Two pathogens change cuticular hydrocarbon profiles but neither elicit a social behavioural change in infected honey bees, Apis mellifera (Apidae: hymenoptera). Austral Entomol 55:147–153
Nelson R, De Renobales M (1987) Chemistry, biochemistry, and physiology of insect cuticular lipids. Arch Insect Biochem Physiol 6:227–265
Nes DN, Lopez M, Zhou W, Guo D, Dowd PF, Norton RA (1997) Sterol utilization and metabolism by Heliothis zea. Lipids 32:1317–1323
Pedrini N, Crespo R, Juárez MP (2007) Biochemistry of insect epicuticle degradation by entomopathogenic fungi. Comp Biochem Physiol C 146:124–137
Roberts DW, St Leger RJS (2004) Metarhizium spp., cosmopolitan insect-pathogenic fungi: mycological aspects. Adv Appl Microbiol 54:1–70
Samuels RI, Paterson IC (1995) Cuticle degrading proteases from insect moulting fluid and culture filtrates of entomopathogenic fungi. Comp Biochem Physiol B 110:661–669
Samuels KDZ, Pinnock DE, Allsopp PG (1989) The potential of Metarhizium anisopliae (metschikoff) sorokin (Deuteromycotina: Hyphomycetes) as a biological control agent of Inopus rubriceps (Macquart) (Diptera: Stratiomyidae). Austr J Entomol 28:69–74
Schal C, Gu X, Burns EL, Blomquist GJ (1994) Patterns of biosynthesis and accumulation of hydrocarbons and contact sex pheromone in the female german cockroach, Blattella germanica. Arch Insect Biochem Physiol 25:375–391
Shah PA, Pell JK (2003) Entomopathogenic fungi as biological control agents. Appl Microbiol Biotechnol 61:413–423
Skalicka-Woźniak K, Los R, Głowniak K, Malm A (2010) Antimicrobial activity of fatty acids from fruits of Peucedanum cervaria and P. alsaticum. Chem Biodivers 7:2748–2754
StatSoft Inc. (2010) STATISTICA (data analysis software system) version 10. Tulsa, Oklahoma
Svoboda JA (1999) Variability of metabolism and function of sterols in insects. Crit Rev Biochem Mol Biol 34:49–57
Svoboda JA, Feldlaufer MF (1991) Neutral sterol metabolism in insects. Lipids 26:614–618
Thomas MB, Jenkins NE (1997) Effects of temperature on growth of Metarhizium flavoviride and virulence to the variegated grasshopper, Zonocerus variegatus. Mycol Res 101:1469–1474
Torrado-León E, Montoya-Lerma J, Valencia-Pizo E (2006) Sublethal effects of Beauveria bassiana (Balsamo) Vuillemin (Deuteromycotina: Hyphomycetes) on the whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) under laboratory conditions. Mycopathologia 162:411–419
Urbanek A, Szadziewski R, Stepnowski P, Boros-Majewska J, Gabriel I, Dawgul M, Kamysz W, Sosnowska D, Gołębiowski M (2012) Composition and antimicrobial activity of fatty acids detected in the hygroscopic secretion collected from the secretory setae of larvae of the biting midge Forcipomyia nigra (Diptera: Ceratopogonidae). J Insect Physiol 58:1265–1276
Wojda I, Kowalski P, Jakubowicz T (2009) Humoral immune response of Galleria mellonella larvae after infection by Beauveria bassiana under optimal and heat-shock conditions. J Insect Physiol 55:525–531
Zheng CJ, Yoo JS, Lee TG, Cho HY, Kim YH, Kim WG (2005) Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett 579:5157–5162
Zimmermann G (1986) Galleria bait method for detection of entomopathogenic fungi in soil. J Appl Entomol 2:213–215
Funding
Financial support was provided by the Polish Ministry of Science and Higher Education under the grant DS 530-8617-D-594-19.
Author information
Authors and Affiliations
Contributions
AU, AB, and MG conceived and designed the study; AU, AP, AB, CT, and MG performed experiments; AU, AN, AB, and MG analyzed the data; AU, AN, PS, and MG interpreted results of experiments; AU, AN, and MG prepared the figures and drafted the manuscript; AU, AN, AB, PS, and MG edited and revised the manuscript; AU,AP, AN, AB, CT, and MG approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Zophobas morio larvae were used for experiments in this article. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Communicated by: Paula Roig Boixeda
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 127 kb)
Rights and permissions
About this article
Cite this article
Gołębiowski, M., Urbanek, A., Pietrzak, A. et al. Effects of the entomopathogenic fungus Metarhizium flavoviride on the fat body lipid composition of Zophobas morio larvae (Coleoptera: Tenebrionidae). Sci Nat 107, 7 (2020). https://doi.org/10.1007/s00114-019-1662-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-019-1662-5