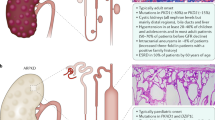
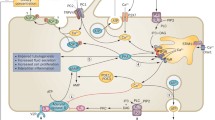
Abstract
Autosomal dominant polycystic kidney disease is a genetic kidney disease caused by mutations in the genes PKD1 or PKD2. Its course is characterized by the formation of progressively enlarged cysts in the renal tubules bilaterally. The basic genetic explanation for autosomal dominant polycystic kidney disease is the double-hit theory, and many of its mechanistic issues can be explained by the cilia doctrine. However, the precise molecular mechanisms underpinning this condition’s occurrence are still not completely understood. Experimental evidence suggests that aquaporins, a class of transmembrane channel proteins, including aquaporin-1, aquaporin-2, aquaporin-3, and aquaporin-11, are involved in the mechanism of autosomal dominant polycystic kidney disease. Aquaporins are either a potential new target for the treatment of autosomal dominant polycystic kidney disease, and further study into the physiopathological role of aquaporins in autosomal dominant polycystic kidney disease will assist to clarify the disease’s pathophysiology and increase the pool of potential treatment options. We primarily cover pertinent findings on aquaporins in autosomal dominant polycystic kidney disease in this review.




Similar content being viewed by others
Availability of data and material
Not applicable.
References
Lanktree MB, Haghighi A, Guiard E, Iliuta IA, Song X, Harris PC, Paterson AD, Pei Y (2018) Prevalence estimates of polycystic kidney and liver disease by population sequencing. J Am Soc Nephrol 29:2593–2600. https://doi.org/10.1681/ASN.2018050493
Willey CJ, Blais JD, Hall AK, Krasa HB, Makin AJ, Czerwiec FS (2017) Prevalence of autosomal dominant polycystic kidney disease in the European Union. Nephrol Dial Transplant 32:1356–1363. https://doi.org/10.1093/ndt/gfw240
Guay-Woodford LM (2003) Murine models of polycystic kidney disease: molecular and therapeutic insights. Am J Physiol Renal Physiol 285:F1034–F1049. https://doi.org/10.1152/ajprenal.00195.2003
Song X, Di Giovanni V, He N, Wang K, Ingram A, Rosenblum ND, Pei Y (2009) Systems biology of autosomal dominant polycystic kidney disease (Adpkd): computational identification of gene expression pathways and integrated regulatory networks. Hum Mol Genet 18:2328–2343. https://doi.org/10.1093/hmg/ddp165
Watnick T, He N, Wang K, Liang Y, Parfrey P, Hefferton D, St George-Hyslop P, Germino G, Pei Y (2000) Mutations of Pkd1 in Adpkd2 cysts suggest a pathogenic effect of trans-heterozygous mutations. Nat Genet 25:143–144. https://doi.org/10.1038/75981
Neumann HP, Jilg C, Bacher J, Nabulsi Z, Malinoc A, Hummel B, Hoffmann MM, Ortiz-Bruechle N, Glasker S, Pisarski P et al (2013) Epidemiology of autosomal-dominant polycystic kidney disease: an in-depth clinical study for South-Western Germany. Nephrol Dial Transplant 28:1472–1487. https://doi.org/10.1093/ndt/gfs551
Torres VE, Harris PC, Pirson Y (2007) Autosomal dominant polycystic kidney disease. Lancet 369:1287–1301. https://doi.org/10.1016/S0140-6736(07)60601-1
Su W, Cao R, Zhang XY, Guan Y (2020) Aquaporins in the kidney: physiology and pathophysiology. Am J Physiol Renal Physiol 318:F193-203. https://doi.org/10.1152/ajprenal.00304.2019
Moeller HB, Fuglsang CH, Fenton RA (2016) Renal aquaporins and water balance disorders. Best Pract Res Clin Endocrinol Metab 30:277–288. https://doi.org/10.1016/j.beem.2016.02.012
Magouliotis DE, Tasiopoulou VS, Svokos AA, Svokos KA (2020) Aquaporins in health and disease. Adv Clin Chem 98:149–171. https://doi.org/10.1016/bs.acc.2020.02.005
He J, Yang B (2019) Aquaporins in renal diseases. Int J Mol Sci 20:366. https://doi.org/10.3390/ijms20020366
Chebib FT, Perrone RD, Chapman AB, Dahl NK, Harris PC, Mrug M, Mustafa RA, Rastogi A, Watnick T, Yu ASL et al (2018) A practical guide for treatment of rapidly progressive Adpkd with tolvaptan. J Am Soc Nephrol 29:2458–2470. https://doi.org/10.1681/ASN.2018060590
Blair HA (2019) Tolvaptan: a review in autosomal dominant polycystic kidney disease. Drugs 79:303–313. https://doi.org/10.1007/s40265-019-1056-1
Harris PC, Ward CJ, Peral B, Hughes J (1995) Autosomal dominant polycystic kidney disease: molecular analysis. Hum Mol Genet 4:1745–1749. https://doi.org/10.1093/hmg/4.suppl_1.1745
Qian F, Watnick TJ, Onuchic LF, Germino GG (1996) The molecular basis of focal cyst formation in human autosomal dominant polycystic kidney disease type I. Cell 87:979–987. https://doi.org/10.1016/s0092-8674(00)81793-6
Brasier JL, Henske EP (1997) Loss of the polycystic kidney disease (Pkd1) region of chromosome 16P13 in renal cyst cells supports a loss-of-function model for cyst pathogenesis. J Clin Invest 99:194–199. https://doi.org/10.1172/JCI119147
Lu W, Shen X, Pavlova A, Lakkis M, Ward CJ, Pritchard L, Harris PC, Genest DR, Perez-Atayde AR, Zhou J (2001) Comparison of Pkd1-targeted mutants reveals that loss of polycystin-1 causes cystogenesis and bone defects. Hum Mol Genet 10:2385–2396. https://doi.org/10.1093/hmg/10.21.2385
Muto S, Aiba A, Saito Y, Nakao K, Nakamura K, Tomita K, Kitamura T, Kurabayashi M, Nagai R, Higashihara E et al (2002) Pioglitazone improves the phenotype and molecular defects of a targeted Pkd1 mutant. Hum Mol Genet 11:1731–1742. https://doi.org/10.1093/hmg/11.15.1731
Wu G, Tian X, Nishimura S, Markowitz GS, D’Agati V, Park JH, Yao L, Li L, Geng L, Zhao H et al (2002) Trans-heterozygous Pkd1 and Pkd2 mutations modify expression of polycystic kidney disease. Hum Mol Genet 11:1845–1854. https://doi.org/10.1093/hmg/11.16.1845
Koptides M, Mean R, Demetriou K, Pierides A, Deltas CC (2000) Genetic evidence for a trans-heterozygous model for cystogenesis in autosomal dominant polycystic kidney disease. Hum Mol Genet 9:447–452. https://doi.org/10.1093/hmg/9.3.447
Pazour GJ (2004) Intraflagellar transport and cilia-dependent renal disease: the ciliary hypothesis of polycystic kidney disease. J Am Soc Nephrol 15:2528–2536. https://doi.org/10.1097/01.ASN.0000141055.57643.E0
Ma M, Tian X, Igarashi P, Pazour GJ, Somlo S (2013) Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nat Genet 45:1004–1012. https://doi.org/10.1038/ng.2715
Hansen JN, Kaiser F, Leyendecker P, Stüven B, Krause JH, Derakhshandeh F, Irfan J, Sroka TJ, Preval KM, Desai PB et al (2022) A camp signalosome in primary cilia drives gene expression and kidney cyst formation. EMBO Rep 23:e54315. https://doi.org/10.15252/embr.202154315
Praetorius HA, Spring KR (2005) A physiological view of the primary cilium. Annu Rev Physiol 67:515–529. https://doi.org/10.1146/annurev.physiol.67.040403.101353
Nauli SM, Zhou J (2004) Polycystins and mechanosensation in renal and nodal cilia. BioEssays 26:844–856. https://doi.org/10.1002/bies.20069
Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB (2002) Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in Orpk mice with polycystic kidney disease. Curr Biol 12:R378–R380. https://doi.org/10.1016/s0960-9822(02)00877-1
Yoder BK, Hou X, Guay-Woodford LM (2002) The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 13:2508–2516. https://doi.org/10.1097/01.asn.0000029587.47950.25
Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ et al (2003) Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33:129–137. https://doi.org/10.1038/ng1076
Hilgendorf KI, Johnson CT, Mezger A, Rice SL, Norris AM, Demeter J, Greenleaf WJ, Reiter JF, Kopinke D, Jackson PK (2019) Omega-3 fatty acids activate ciliary ffar4 to control adipogenesis. Cell 179:1289–1305. https://doi.org/10.1016/j.cell.2019.11.005
Siljee JE, Wang Y, Bernard AA, Ersoy BA, Zhang S, Marley A, Von Zastrow M, Reiter JF, Vaisse C (2018) Subcellular localization of Mc4R with Adcy3 at neuronal primary cilia underlies a common pathway for genetic predisposition to obesity. Nat Genet 50:180–185. https://doi.org/10.1038/s41588-017-0020-9
DeCaen PG, Delling M, Vien TN, Clapham DE (2013) Direct recording and molecular identification of the calcium channel of primary cilia. Nature 504:315–318. https://doi.org/10.1038/nature12832
Pala R, Alomari N, Nauli SM (2017) Primary cilium-dependent signaling mechanisms. Int J Mol Sci 18:2272. https://doi.org/10.3390/ijms18112272
Ko JY (2016) Functional study of the primary cilia in Adpkd. Adv Exp Med Biol 933:45–57. https://doi.org/10.1007/978-981-10-2041-4_5
Low SH, Vasanth S, Larson CH, Mukherjee S, Sharma N, Kinter MT, Kane ME, Obara T, Weimbs T (2006) Polycystin-1, Stat6, and P100 function in a pathway that transduces ciliary mechanosensation and is activated in polycystic kidney disease. Dev Cell 10:57–69. https://doi.org/10.1016/j.devcel.2005.12.005
Nauli SM, Rossetti S, Kolb RJ, Alenghat FJ, Consugar MB, Harris PC, Ingber DE, Loghman-Adham M, Zhou J (2006) Loss of polycystin-1 in human cyst-lining epithelia leads to ciliary dysfunction. J Am Soc Nephrol 17:1015–1025. https://doi.org/10.1681/ASN.2005080830
Huang L, Lipschutz JH (2014) Cilia and polycystic kidney disease, kith and kin. Birth Defects Res C Embryo Today 102:174–185. https://doi.org/10.1002/bdrc.21066
Delling M, DeCaen PG, Doerner JF, Febvay S, Clapham DE (2013) Primary cilia are specialized calcium signalling organelles. Nature 504:311–314. https://doi.org/10.1038/nature12833
Delling M, Indzhykulian AA, Liu X, Li Y, Xie T, Corey DP, Clapham DE (2016) Primary cilia are not calcium-responsive mechanosensors. Nature 531:656–660. https://doi.org/10.1038/nature17426
Shao L, El-Jouni W, Kong F, Ramesh J, Kumar RS, Shen X, Ren J, Devendra S, Dorschel A, Wu M et al (2020) Genetic reduction of cilium length by targeting intraflagellar transport 88 protein impedes kidney and liver cyst formation in mouse models of autosomal polycystic kidney disease. Kidney Int 98:1225–1241. https://doi.org/10.1016/j.kint.2020.05.049
Sharma N, Malarkey EB, Berbari NF, O’Connor AK, Vanden Heuvel GB, Mrug M, Yoder BK (2013) Proximal tubule proliferation is insufficient to induce rapid cyst formation after cilia disruption. J Am Soc Nephrol 24:456–464. https://doi.org/10.1681/ASN.2012020154
Ma M, Gallagher AR, Somlo S (2017) Ciliary mechanisms of cyst formation in polycystic kidney disease. Cold Spring Harb Perspect Biol 9:a028209. https://doi.org/10.1101/cshperspect.a028209
Luo L, Roy S, Li L, Ma M (2023) Polycystic kidney disease: novel insights into polycystin function. Trends Mol Med 29:268–281. https://doi.org/10.1016/j.molmed.2023.01.005
Ma M (2021) Cilia and polycystic kidney disease. Semin Cell Dev Biol 110:139–148. https://doi.org/10.1016/j.semcdb.2020.05.003
Hopp K, Ward CJ, Hommerding CJ, Nasr SH, Tuan HF, Gainullin VG, Rossetti S, Torres VE, Harris PC (2012) Functional polycystin-1 dosage governs autosomal dominant polycystic kidney disease severity. J Clin Invest 122:4257–4273. https://doi.org/10.1172/JCI64313
Fedeles SV, Tian X, Gallagher AR, Mitobe M, Nishio S, Lee SH, Cai Y, Geng L, Crews CM, Somlo S (2011) A genetic interaction network of five genes for human polycystic kidney and liver diseases defines polycystin-1 as the central determinant of cyst formation. Nat Genet 43:639–647. https://doi.org/10.1038/ng.860
Torres VE, Harris PC (2014) Strategies targeting camp signaling in the treatment of polycystic kidney disease. J Am Soc Nephrol 25:18–32. https://doi.org/10.1681/ASN.2013040398
Chebib FT, Sussman CR, Wang X, Harris PC, Torres VE (2015) Vasopressin and disruption of calcium signalling in polycystic kidney disease. Nat Rev Nephrol 11:451–464. https://doi.org/10.1038/nrneph.2015.39
Mick DU, Rodrigues RB, Leib RD, Adams CM, Chien AS, Gygi SP, Nachury MV (2015) Proteomics of primary cilia by proximity labeling. Dev Cell 35:497–512. https://doi.org/10.1016/j.devcel.2015.10.015
Bachmann VA, Mayrhofer JE, Ilouz R, Tschaikner P, Raffeiner P, Röck R, Courcelles M, Apelt F, Lu TW, Baillie GS et al (2016) Gpr161 anchoring of Pka consolidates Gpcr and camp signaling. Proc Natl Acad Sci U S A 113:7786–7791. https://doi.org/10.1073/pnas.1608061113
Mykytyn K, Askwith C (2017) Askwith. G-protein-coupled receptor signaling in cilia. Cold Spring Harb Perspect Biol 9:a028183. https://doi.org/10.1101/cshperspect.a028183
Wachten D, Mick DU (2021) Signal transduction in primary cilia - analyzing and manipulating gpcr and second messenger signaling. Pharmacol Ther 224:107836. https://doi.org/10.1016/j.pharmthera.2021.107836
Somatilaka BN, Hwang SH, Palicharla VR, White KA, Badgandi H, Shelton JM, Mukhopadhyay S (2020) Ankmy2 prevents smoothened-independent hyperactivation of the hedgehog pathway via cilia-regulated adenylyl cyclase signaling. Dev Cell 54:710–726. https://doi.org/10.1016/j.devcel.2020.06.034
Wang Y, Bernard A, Comblain F, Yue X, Paillart C, Zhang S, Reiter JF, Vaisse C (2021) Melanocortin 4 receptor signals at the neuronal primary cilium to control food intake and body weight. J Clin Invest 131:e142064. https://doi.org/10.1172/JCI142064
Reiterová J, Tesař V (2022) Autosomal dominant polycystic kidney disease: from pathophysiology of cystogenesis to advances in the treatment. Int J Mol Sci 23:3317. https://doi.org/10.3390/ijms23063317
Li C, Wang W (2017) Molecular biology of aquaporins. Adv Exp Med Biol 969:1–34. https://doi.org/10.1007/978-94-024-1057-0_1
Verkman AS (2012) Aquaporins in clinical medicine. Annu Rev Med 63:303–316. https://doi.org/10.1146/annurev-med-043010-193843
Kourghi M, Pei JV, De Ieso ML, Nourmohammadi S, Chow PH, Yool AJ (2018) Fundamental structural and functional properties of aquaporin ion channels found across the kingdoms of life. Clin Exp Pharmacol Physiol 45:401–409. https://doi.org/10.1111/1440-1681.12900
Geng X, Yang B (2017) Transport characteristics of aquaporins. Adv Exp Med Biol 969:51–62. https://doi.org/10.1007/978-94-024-1057-0_3
Ishibashi K (2009) New members of mammalian aquaporins: Aqp10-Aqp12. Handb Exp Pharmacol 190:251–262. https://doi.org/10.1007/978-3-540-79885-9_13
Ishibashi K, Tanaka Y, Morishita Y (2021) The role of mammalian superaquaporins inside the cell: an update. Biochimica et Biophysica Acta (BBA) - Biomembranes 1863:183617. https://doi.org/10.1016/j.bbamem.2021.183617
Verani RR, Silva FG (1988) Histogenesis of the renal cysts in adult (autosomal dominant) polycystic kidney disease: a histochemical study. Mod Pathol 1:457–463
Raphael KL, Strait KA, Stricklett PK, Miller RL, Nelson RD, Piontek KB, Germino GG, Kohan DE (2009) Inactivation of Pkd1 in principal cells causes a more severe cystic kidney disease than in intercalated cells. Kidney Int 75:626–633. https://doi.org/10.1038/ki.2008.659
Loghman-Adham M, Nauli SM, Soto CE, Kariuki B, Zhou J (2003) Immortalized epithelial cells from human autosomal dominant polycystic kidney cysts. Am J Physiol Renal Physiol 285:F397-412. https://doi.org/10.1152/ajprenal.00310.2002
Tsukiyama T, Kobayashi K, Nakaya M, Iwatani C, Seita Y, Tsuchiya H, Matsushita J, Kitajima K, Kawamoto I, Nakagawa T et al (2019) Monkeys mutant for PKD1 recapitulate human autosomal dominant polycystic kidney disease. Nat Commun 10:5517. https://doi.org/10.1038/s41467-019-13398-6
Ishibashi K, Tanaka Y, Morishita Y (2020) Perspectives on the evolution of aquaporin superfamily. Vitam Horm 112:1–27. https://doi.org/10.1016/bs.vh.2019.08.001
akata K, Tani K, Fujiyoshi Y, (2011) Water permeability and characterization of aquaporin-11. J Struct Biol 174:315–320. https://doi.org/10.1016/j.jsb.2011.01.003
Morishita Y, Matsuzaki T, Hara-chikuma M, Andoo A, Shimono M, Matsuki A, Kobayashi K, Ikeda M, Yamamoto T, Verkman A et al (2005) Disruption of aquaporin-11 produces polycystic kidneys following vacuolization of the proximal tubule. Mol Cell Biol 25:7770–7779. https://doi.org/10.1128/MCB.25.17.7770-7779.2005
Elkjaer ML, Nejsum LN, Gresz V, Kwon TH, Jensen UB, Frøkiaer J, Nielsen S (2001) Immunolocalization of aquaporin-8 in rat kidney, gastrointestinal tract, testis, and airways. Am J Physiol Renal Physiol 281:F1047–F1057. https://doi.org/10.1152/ajprenal.00158.2001
Rützler M, Rojek A, Damgaard MV, Andreasen A, Fenton RA, Nielsen S (2017) Temporal deletion of aqp11 in mice is linked to the severity of cyst-like disease. American Journal of Physiology-Renal Physiology 312:F343–F351. https://doi.org/10.1152/ajprenal.00065.2016
Torres VE, Harris PC (2006) Mechanisms of disease: autosomal dominant and recessive polycystic kidney diseases. Nat Clin Pract Nephrol 2:40–55. https://doi.org/10.1038/ncpneph0070
Okada S, Misaka T, Tanaka Y, Matsumoto I, Ishibashi K, Sasaki S, Abe K (2008) Aquaporin-11 knockout mice and polycystic kidney disease animals share a common mechanism of cyst formation. FASEB J 22:3672–3684. https://doi.org/10.1096/fj.08-111872
Han CL, Chien CW, Chen WC, Chen YR, Wu CP, Li H, Chen YJ (2008) A multiplexed quantitative strategy for membrane proteomics: opportunities for mining therapeutic targets for autosomal dominant polycystic kidney disease. Mol Cell Proteomics 7:1983–1997. https://doi.org/10.1074/mcp.M800068-MCP200
Saito T, Tanaka Y, Morishita Y, Ishibashi K (2018) Proteomic analysis of Aqp11-null kidney: proximal tubular type polycystic kidney disease. Biochem Biophys Rep 13:17–21. https://doi.org/10.1016/j.bbrep.2017.11.003
Arrell DK, Elliott ST, Kane LA, Guo Y, Ko YH, Pedersen PL, Robinson J, Murata M, Murphy AM, Marbán E et al (2006) Proteomic analysis of pharmacological preconditioning: novel protein targets converge to mitochondrial metabolism pathways. Circ Res 99:706–714. https://doi.org/10.1161/01.RES.0000243995.74395.f8
Kobayashi A, Goto Y, Nagata M, Yamaguchi Y (2010) Granular swollen epithelial cells: a histologic and diagnostic marker for mitochondrial nephropathy. Am J Surg Pathol 34:262–270. https://doi.org/10.1097/PAS.0b013e3181cb4ed3
Podrini C, Cassina L, Boletta A (2020) Metabolic reprogramming and the role of mitochondria in polycystic kidney disease. Cell Signal 67:109495. https://doi.org/10.1016/j.cellsig.2019.109495
Bestetti S, Galli M, Sorrentino I, Pinton P, Rimessi A, Sitia R, Medraño-Fernandez I (2020) Human aquaporin-11 guarantees efficient transport of H(2)O(2) across the endoplasmic reticulum membrane. Redox Biol 28:101326. https://doi.org/10.1016/j.redox.2019.101326
Yoboue ED, Sitia R, Simmen T (2018) Redox crosstalk at endoplasmic reticulum (Er) membrane contact sites (Mcs) uses toxic waste to deliver messages. Cell Death Dis 9:331. https://doi.org/10.1038/s41419-017-0033-4
Sorrentino I, Galli M, Medraño-Fernandez I, Sitia R (2022) Transfer of H(2)O(2) From mitochondria to the endoplasmic reticulum via aquaporin-11. Redox Biol 55:102410. https://doi.org/10.1016/j.redox.2022.102410
Karala AR, Lappi AK, Saaranen MJ, Ruddock LW (2009) Efficient peroxide-mediated oxidative refolding of a protein at physiological Ph and implications for oxidative folding in the endoplasmic reticulum. Antioxid Redox Signal 11:963–970. https://doi.org/10.1089/ars.2008.2326
Ahner A, Brodsky JL (2004) Checkpoints in Er-associated degradation: excuse me, which way to the proteasome? Trends Cell Biol 14:474–478. https://doi.org/10.1016/j.tcb.2004.07.013
Inoue Y, Sohara E, Kobayashi K, Chiga M, Rai T, Ishibashi K, Horie S, Su X, Zhou J, Sasaki S et al (2014) Aberrant glycosylation and localization of polycystin-1 cause polycystic kidney in an Aqp11 knockout model. J Am Soc Nephrol 25:2789–2799. https://doi.org/10.1681/ASN.2013060614
Nielsen S et al (1993) Chip28 water channels are localized in constitutively water-permeable segments of the nephron. J Cell Biol 120(2):371–383. https://doi.org/10.1083/jcb.120.2.371
Anthony TL, Brooks HL, Boassa D, Leonov S, Yanochko GM, Regan JW, Yool AJ (2000) Cloned human aquaporin-1 is a cyclic gmp-gated ion channel. Mol Pharmacol 57:576–588. https://doi.org/10.1124/mol.57.3.576
Yool AJ, Stamer WD, Regan JW (1996) Forskolin stimulation of water and cation permeability in aquaporin 1 water channels. Science 273:1216–1218. https://doi.org/10.1126/science.273.5279.1216
Belkacemi L, Beall MH, Magee TR, Pourtemour M, Ross MG (2008) Aqp1 gene expression is upregulated by arginine vasopressin and cyclic amp agonists in trophoblast cells. Life Sci 82:1272–1280. https://doi.org/10.1016/j.lfs.2008.04.014
Bachinsky DR, Sabolic I, Emmanouel DS, Jefferson DM, Carone FA, Brown D, Perrone RD (1995) Water channel expression in human ADPKD kidneys. Am J Physiol 268:F398. https://doi.org/10.1152/ajprenal.1995.268.3.F398
Li D, Shi X, Zhao L, Liang Z, Xie S, Wang G (2016) Overexpression of aquaporin 1 on cysts of patients with polycystic liver disease. Rev Esp Enferm Dig 2108:71–78. https://doi.org/10.17235/reed.2015.3960/2015
Chen Y, Tachibana O, Oda M, Xu R, Hamada J, Yamashita J, Hashimoto N, Takahashi JA (2006) Increased expression of aquaporin 1 in human hemangioblastomas and its correlation with cyst formation. J Neurooncol 80:219–225. https://doi.org/10.1007/s11060-005-9057-1
Devuyst O, Burrow CR, Smith BL, Agre P, Knepper MA, Wilson PD (1996) Expression of aquaporins-1 and -2 during nephrogenesis and in autosomal dominant polycystic kidney disease. Am J Physiol 271:F169–F183. https://doi.org/10.1152/ajprenal.1996.271.1.F169
Echevarría M, Muñoz-Cabello AM, Sánchez-Silva R, Toledo-Aral JJ, López-Barneo J (2007) Development of cytosolic hypoxia and hypoxia-inducible factor stabilization are facilitated by aquaporin-1 expression. J Biol Chem 282:30207–30215. https://doi.org/10.1074/jbc.M702639200
Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Hicklin DJ, Huber J, Nakatani T, Tsujinoue H, Yanase K (2002) Synergistic effect of basic fibroblast growth factor and vascular endothelial growth factor in murine hepatocellular carcinoma. Hepatology 35:834–842. https://doi.org/10.1053/jhep.2002.32541
Lin Y, Wei J, Zhang Y, Huang J, Wang S, Luo Q, Yu H, Ji L, Zhou X, Li C (2023) Shen Qi Wan attenuates renal interstitial fibrosis through upregulating AQP1. Chin J Nat Med 21:359–370. https://doi.org/10.1016/S1875-5364(23)60453-4
Hara-Chikuma M, Verkman AS (2006) Aquaporin-1 facilitates epithelial cell migration in kidney proximal tubule. J Am Soc Nephrol 17:39–45. https://doi.org/10.1681/ASN.2005080846
Fu Y, Kim I, Lian P, Li A, Zhou L, Li C, Liang D, Coffey RJ, Ma J, Zhao P et al (2010) Loss of Bicc1 impairs tubulomorphogenesis of cultured IMCD cells by disrupting E-cadherin-based cell-cell adhesion. Eur J Cell Biol 89:428–436. https://doi.org/10.1016/j.ejcb.2010.01.002
Wang W, Li F, Sun Y, Lei L, Zhou H, Lei T, Xia Y, Verkman AS, Yang B (2015) Aquaporin-1 retards renal cyst development in polycystic kidney disease by inhibition of Wnt signaling. FASEB J 29:1551–1563. https://doi.org/10.1096/fj.14-260828
Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA (1995) Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-Cd water channels to plasma membrane. Proc Natl Acad Sci U S A 92:1013–1017. https://doi.org/10.1073/pnas.92.4.1013
Noda Y, Sasaki S (2021) Updates and perspectives on aquaporin-2 and water balance disorders. Int J Mol Sci 22:12950. https://doi.org/10.3390/ijms222312950
Lolait SJ, O’Carroll AM, McBride OW, Konig M, Morel A, Brownstein MJ (1992) Cloning and characterization of a vasopressin V2 receptor and possible link to nephrogenic diabetes insipidus. Nature 357:336–339. https://doi.org/10.1038/357336a0
Birnbaumer M, Seibold A, Gilbert S, Ishido M, Barberis C, Antaramian A, Brabet P, Rosenthal W (1992) Molecular cloning of the receptor for human antidiuretic hormone. Nature 357:333–335. https://doi.org/10.1038/357333a0
Isobe K, Jung HJ, Yang CR, Claxton J, Sandoval P, Burg MB, Raghuram V, Knepper MA (2017) Systems-level identification of Pka-dependent signaling in epithelial cells. Proc Natl Acad Sci U S A 114:E8875–E8884. https://doi.org/10.1073/pnas.1709123114
Seeman T, Dusek J, Vondrák K, Bláhová K, Simková E, Kreisinger J, Dvorák P, Kyncl M, Hríbal Z, Janda J (2004) Renal concentrating capacity is linked to blood pressure in children with autosomal dominant polycystic kidney disease. Physiol Res 53(6):629–634. (PMID: 15588131)
Morgenthaler NG, Struck J, Alonso C, Bergmann A (2006) Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 52:112–119. https://doi.org/10.1373/clinchem.2005.060038
Struck J, Morgenthaler NG, Bergmann A (2005) Copeptin, a stable peptide derived from the vasopressin precursor, is elevated in serum of sepsis patients. Peptides 26:2500–2504. https://doi.org/10.1016/j.peptides.2005.04.019
Boertien WE, Meijer E, Li J, Bost JE, Struck J, Flessner MF, Gansevoort RT, Torres VE; Consortium for radiologic imaging studies of polycystic kidney disease CRISP (2013) Relationship of copeptin, a surrogate marker for arginine vasopressin, with change in total kidney volume and Gfr decline in autosomal dominant polycystic kidney disease: results from the crisp cohort. Am J Kidney Dis 61:420–429. https://doi.org/10.1053/j.ajkd.2012.08.038
Meijer E, Bakker SJ, van der Jagt EJ, Navis G, de Jong PE, Struck J, Gansevoort RT (2011) Copeptin, a surrogate marker of vasopressin, is associated with disease severity in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 6:361–368. https://doi.org/10.2215/CJN.04560510
Boertien WE, Meijer E, Zittema D, van Dijk MA, Rabelink TJ, Breuning MH, Struck J, Bakker SJ, Peters DJ, de Jong PE et al (2012) Copeptin, a surrogate marker for vasopressin, is associated with kidney function decline in subjects with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 27:4131–4137. https://doi.org/10.1093/ndt/gfs070
van Gastel MDA, Torres VE (2017) Polycystic kidney disease and the vasopressin pathway. Ann Nutr Metab 70:43–50. https://doi.org/10.1159/000463063
Wang X, Wu Y, Ward CJ, Harris PC, Torres VE (2008) Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol 19:102–108. https://doi.org/10.1681/ASN.2007060688
Gattone VH 2nd, Wang X, Harris PC, Torres VE (2003) Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 9:1323–1326. https://doi.org/10.1038/nm935
Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS et al (2012) Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367:2407–2418. https://doi.org/10.1056/NEJMoa1205511
Boertien WE, Meijer E, de Jong PE, ter Horst GJ, Renken RJ, van der Jagt EJ, Kappert P, Ouyang J, Engels GE, van Oeveren W et al (2015) Short-term effects of tolvaptan in individuals with autosomal dominant polycystic kidney disease at various levels of kidney function. Am J Kidney Dis 65:833–841. https://doi.org/10.1053/j.ajkd.2014.11.010
Devuyst O, Chapman AB, Gansevoort RT, Higashihara E, Perrone RD, Torres VE, Blais JD, Zhou W, Ouyang J, Czerwiec FS (2017) Urine osmolality, response to tolvaptan, and outcome in autosomal dominant polycystic kidney disease: results from the TEMPO 3:4 trial. J Am Soc Nephrol 28(5):1592–1602. https://doi.org/10.1681/ASN.2016040448
Aboudehen K, Noureddine L, Cobo-Stark P, Avdulov S, Farahani S, Gearhart MD, Bichet DG, Pontoglio M, Patel V, Igarashi P (2017) Hepatocyte nuclear factor-1beta regulates urinary concentration and response to hypertonicity. J Am Soc Nephrol 28:2887–2900. https://doi.org/10.1681/ASN.2016101095
Noitem R, Yuajit C, Soodvilai S, Muanprasat C, Chatsudthipong V (2018) Steviol slows renal cyst growth by reducing Aqp2 expression and promoting Aqp2 degradation. Biomed Pharmacother 101:754–762. https://doi.org/10.1016/j.biopha.2018.02.139
Chen Y, Rice W, Gu Z, Li J, Huang J, Brenner MB, Van Hoek A, Xiong J, Gundersen GG, Norman JC et al (2012) Aquaporin 2 promotes cell migration and epithelial morphogenesis. J Am Soc Nephrol 23(9):1506–1517. https://doi.org/10.1681/ASN.2012010079
Ando F, Sohara E, Morimoto T, Yui N, Nomura N, Kikuchi E, Takahashi D, Mori T, Vandewalle A, Rai T et al (2016) Wnt5a induces renal Aqp2 expression by activating calcineurin signalling pathway. Nat Commun 7:13636. https://doi.org/10.1038/ncomms13636
Tamma G, Lasorsa D, Trimpert C, Ranieri M, Di Mise A, Mola MG, Mastrofrancesco L, Devuyst O, Svelto M, Deen PM et al (2014) A protein kinase A-independent pathway controlling aquaporin 2 trafficking as a possible cause for the syndrome of inappropriate antidiuresis associated with polycystic kidney disease 1 haploinsufficiency. J Am Soc Nephrol 25:2241–2253. https://doi.org/10.1681/ASN.2013111234
Jang HJ, Park HJ, Choi HS, Jung HJ, Kwon TH (2023) Genome-engineered mpkCCDc14 cells as a new resource for studying AQP2. Int J Mol Sci 24:1684. https://doi.org/10.3390/ijms24021684
Wang Y, Chen D, Liu Y, Zhang Y, Duan C, Otkur W, Chen H, Liu X, Xia T, Qi H et al (2021) Aqp3-Mediated H(2) O(2) Uptake inhibits luad autophagy by inactivating Pten. Cancer Sci 112(8):3278–3292. https://doi.org/10.1111/cas.15008
Yang B, Verkman VAS (1997) Water and glycerol permeabilities of aquaporins 1–5 and mip determined quantitatively by expression of epitope-tagged constructs in Xenopus oocytes. J Biol Chem 272:16140–16146. https://doi.org/10.1074/jbc.272.26.16140
Ishibashi K, Sasaki S, Fushimi K, Uchida S, Kuwahara M, Saito H, Furukawa T, Nakajima K, Yamaguchi Y, Gojobori T et al (1994) Molecular cloning and expression of a member of the aquaporin family with permeability to glycerol and urea in addition to water expressed at the basolateral membrane of kidney collecting duct cells. Proc Natl Acad Sci U S A 91:6269–6273. https://doi.org/10.1073/pnas.91.14.6269
Nielsen S, Frøkiaer J, Marples D, Kwon TH, Agre P, Knepper MA (2002) Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82:205–244. https://doi.org/10.1152/physrev.00024.2001
Terris J, Ecelbarger CA, Nielsen S, Knepper MA (1996) Long-term regulation of four renal aquaporins in rats. Am J Physiol 271:F414–F422. https://doi.org/10.1152/ajprenal.1996.271.2.F414
Matsuzaki T, Suzuki T, Takata K (2001) Hypertonicity-induced expression of aquaporin 3 in Mdck cells. Am J Physiol Cell Physiol 281:C55-63. https://doi.org/10.1152/ajpcell.2001.281.1.C55
Marlar S, Arnspang EC, Koffman JS, Løcke EM, Christensen BM, Nejsum LN (2014) Elevated camp increases aquaporin-3 plasma membrane diffusion. Am J Physiol Cell Physiol 306:C598-606. https://doi.org/10.1152/ajpcell.00132.2013
Miller EW, Dickinson BC, Chang CJ (2010) Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc Natl Acad Sci U S A 107:15681–15686. https://doi.org/10.1073/pnas.1005776107
Hara-Chikuma M, Satooka H, Watanabe S, Honda T, Miyachi Y, Watanabe T, Verkman AS (2015) Aquaporin-3-mediated hydrogen peroxide transport is required for Nf-Kappab signalling in keratinocytes and development of psoriasis. Nat Commun 6:7454. https://doi.org/10.1038/ncomms8454
Rowe I, Chiaravalli M, Mannella V, Ulisse V, Quilici G, Pema M, Song XW, Xu H, Mari S, Qian F et al (2013) Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat Med 19:488–493. https://doi.org/10.1038/nm.3092
Menezes LF, Germino GG (2019) The pathobiology of polycystic kidney disease from a metabolic viewpoint. Nat Rev Nephrol 15:735–749. https://doi.org/10.1038/s41581-019-0183-y
Wang W, Geng X, Lei L, Jia Y, Li Y, Zhou H, Verkman AS, Yang B (2019) Aquaporin-3 deficiency slows cyst enlargement in experimental mouse models of autosomal dominant polycystic kidney disease. FASEB J 33:6185–6196. https://doi.org/10.1096/fj.201801338RRR
Wang J, Whiteman MW, Lian H, Wang G, Singh A, Huang D, Denmark T (2009) A non-canonical Mek/Erk signaling pathway regulates autophagy via regulating Beclin 1. J Biol Chem 284:21412–21424. https://doi.org/10.1074/jbc.M109.026013
Ibraghimov-Beskrovnaya O, Natoli TA (2011) mTOR signaling in polycystic kidney disease. Trends Mol Med 17:625–633. https://doi.org/10.1016/j.molmed.2011.06.003
Chou CL, Ma T, Yang B, Knepper MA, Verkman AS (1998) Fourfold reduction of water permeability in inner medullary collecting duct of aquaporin-4 knockout mice. Am J Physiol 274:C549–C554. https://doi.org/10.1152/ajpcell.1998.274.2.C549
Ma T, Song Y, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS (2000) Nephrogenic diabetes insipidus in mice lacking aquaporin-3 water channels. Proc Natl Acad Sci U S A 97:4386–4391. https://doi.org/10.1073/pnas.080499597
Ishibashi K, Kuwahara M, Gu Y, Kageyama Y, Tohsaka A, Suzuki F, Marumo F, Sasaki S (1997) Cloning and functional expression of a new water channel abundantly expressed in the testis permeable to water, glycerol, and urea. J Biol Chem 272:20782–20786. https://doi.org/10.1074/jbc.272.33.20782
Ma Y, Zhang J, Li Y, Hu H, Ye Q, Yang C, Yang L, Zhang B, Ma T (2023) Aquaporin-7 facilitates proliferation and adipogenic differentiation of mouse bone marrow mesenchymal stem cells by regulating hydrogen peroxide transport. Stem Cell Rev Rep 19:2378–2390. https://doi.org/10.1007/s12015-023-10588-0
Author information
Authors and Affiliations
Contributions
Qiumei Lan and Jie Li conceived and designed the original draft. Hanqing Zhang, Zijun Zhou, and Yaxuan Fang prepared the manuscript. Bo Yang reviewed and edited the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lan, Q., Li, J., Zhang, H. et al. Mechanistic complement of autosomal dominant polycystic kidney disease: the role of aquaporins. J Mol Med 102, 773–785 (2024). https://doi.org/10.1007/s00109-024-02446-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-024-02446-4