Abstract
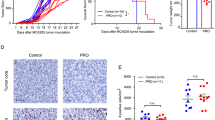
Preclinical and early clinical mechanistic studies of antitumor activity from the beta-adrenergic receptor (β-AR) blocker propranolol have revealed both cell signaling and immune function pathway effects. Intertumoral studies were performed using propranolol, a β1-AR selective agent (atenolol), and a β2-AR selective agent (ICI 118,551) in a preclinical in vivo model, as a step to dissect the contribution of cell signaling and CD8+ immunological effects on anticancer activity. We found that repression of β2-AR but not β1-AR signaling selectively suppressed cell viability and inhibited xenograft growth in vivo. Moreover, western blot analysis indicated that the phosphorylation levels of AKT/MEK/ERK were significantly decreased following the inhibition of β2-AR. Furthermore, propranolol was found to activate the tumor microenvironment by inducing an increased intratumoral frequency of CD8+ T cells, whereas neither selective β1 nor β2-AR blockers had a significant effect on the tumor immune microenvironment. Thus, the results of this mechanistic dissection support a predominant role of tumor cell signaling, rather than the accumulation of CD8+ T cells, as the basis for propranolol antitumor activity.
Key messages
-
Molecular signaling of AKT/MAPK pathway contributes to propranolol caused cancer control.
-
CD8+ T cells in tumor microenvironment were activated upon propranolol exposure.
-
The basis for propranolol antitumor activity was predominantly dependent on cell signaling, rather than the activation of CD8+ T cells.




Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
He RH, He YJ, Tang YJ, Zhou HH, McLeod HL, Liu J (2016) The potential anticancer effect of beta-blockers and the genetic variations involved in the interindividual difference. Pharmacogenomics 17(1):74–79
Shahrokhi M, Gupta V (2020) Propranolol. StatPearls, Treasure Island (FL)
Oliver E, Mayor F Jr, D’Ocon P (2019) Beta-blockers: historical perspective and mechanisms of action. Rev Esp Cardiol (Engl Ed) 72(10):853–862
Hagen R, Ghareeb E, Jalali O, Zinn Z (2018) Infantile hemangiomas: what have we learned from propranolol? Curr Opin Pediatr 30(4):499–504
Perron L, Bairati I, Harel F, Meyer F (2004) Antihypertensive drug use and the risk of prostate cancer (Canada). Cancer Causes Control 15(6):535–541
Powe DG, Voss MJ, Zanker KS, Habashy HO, Green AR, Ellis IO et al (2010) Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 1(7):628–638
De Giorgi V, Gandini S, Grazzini M, Benemei S, Marchionni N, Geppetti P (2013) Effect of beta-blockers and other antihypertensive drugs on the risk of melanoma recurrence and death. Mayo Clin Proc 88(11):1196–1203
Liao P, Song K, Zhu Z, Liu Z, Zhang W, Li W et al (2020) Propranolol suppresses the growth of colorectal cancer through simultaneously activating autologous CD8(+) T cells and inhibiting tumor AKT/MAPK pathway. Clin Pharmacol Ther 108(3):606–615
Zhou C, Chen X, Zeng W, Peng C, Huang G, Li X et al (2016) Propranolol induced G0/G1/S phase arrest and apoptosis in melanoma cells via AKT/MAPK pathway. Oncotarget 7(42):68314–68327
De Giorgi V, Grazzini M, Benemei S, Marchionni N, Botteri E, Pennacchioli E et al (2018) Propranolol for off-label treatment of patients with melanoma: results from a cohort study. JAMA Oncol 4(2):e172908
Killock D (2017) Skin cancer: propranolol limits melanoma recurrence. Nat Rev Clin Oncol 14(12):714
Hiller JG, Cole SW, Crone EM, Byrne DJ, Shackleford DM, Pang JB et al (2020) Preoperative beta-blockade with propranolol reduces biomarkers of metastasis in breast cancer: a phase II randomized trial. Clin Cancer Res 26(8):1803–1811
Spini A, Roberto G, Gini R, Bartolini C, Bazzani L, Donnini S et al (2019) Evidence of beta-blockers drug repurposing for the treatment of triple negative breast cancer: a systematic review. Neoplasma 66(6):963–970
Ramondetta LM, Hu W, Thaker PH, Urbauer DL, Chisholm GB, Westin SN et al (2019) Prospective pilot trial with combination of propranolol with chemotherapy in patients with epithelial ovarian cancer and evaluation on circulating immune cell gene expression. Gynecol Oncol 154(3):524–530
Yang EV, Kim SJ, Donovan EL, Chen M, Gross AC, Webster Marketon JI et al (2009) Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: implications for stress-related enhancement of tumor progression. Brain Behav Immun 23(2):267–275
Moretti S, Massi D, Farini V, Baroni G, Parri M, Innocenti S et al (2013) Beta-adrenoceptors are upregulated in human melanoma and their activation releases pro-tumorigenic cytokines and metalloproteases in melanoma cell lines. Lab Invest 93(3):279–290
Inigo-Marco I, Alonso MM (2019) Destress and do not suppress: targeting adrenergic signaling in tumor immunosuppression. J Clin Invest 129(12):5086–5088
Hu Q, Liao P, Li W, Hu J, Chen C, Zhang Y et al (2021) Clinical use of propranolol reduces biomarkers of proliferation in gastric cancer. Front Oncol 11:628613
Montoya A, Varela-Ramirez A, Dickerson E, Pasquier E, Torabi A, Aguilera R et al (2019) The beta adrenergic receptor antagonist propranolol alters mitogenic and apoptotic signaling in late stage breast cancer. Biomed J 42(3):155–165
Masur K, Niggemann B, Zanker KS, Entschladen F (2001) Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res 61(7):2866–2869
Sood AK, Bhatty R, Kamat AA, Landen CN, Han L, Thaker PH et al (2006) Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res 12(2):369–375
Guo K, Ma Q, Wang L, Hu H, Li J, Zhang D et al (2009) Norepinephrine-induced invasion by pancreatic cancer cells is inhibited by propranolol. Oncol Rep 22(4):825–830
Mohammadpour H, MacDonald CR, Qiao G, Chen M, Dong B, Hylander BL et al (2019) beta2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J Clin Invest 129(12):5537–5552
Zhang X, Zhang Y, He Z, Yin K, Li B, Zhang L et al (2019) Chronic stress promotes gastric cancer progression and metastasis: an essential role for ADRB2. Cell Death Dis 10(11):788
Bucsek MJ, Qiao G, MacDonald CR, Giridharan T, Evans L, Niedzwecki B et al (2017) Beta-adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in CD8(+) T cells and undermines checkpoint inhibitor therapy. Cancer Res 77(20):5639–5651
Qiao G, Bucsek MJ, Winder NM, Chen M, Giridharan T, Olejniczak SH et al (2019) beta-Adrenergic signaling blocks murine CD8(+) T-cell metabolic reprogramming during activation: a mechanism for immunosuppression by adrenergic stress. Cancer Immunol Immunother 68(1):11–22
Estrada LD, Agac D, Farrar JD (2016) Sympathetic neural signaling via the beta2-adrenergic receptor suppresses T-cell receptor-mediated human and mouse CD8(+) T-cell effector function. Eur J Immunol 46(8):1948–1958
Yeo W, Chan SL, Mo FK, Chu CM, Hui JW, Tong JH et al (2015) Phase I/II study of temsirolimus for patients with unresectable Hepatocellular Carcinoma (HCC)- a correlative study to explore potential biomarkers for response. BMC Cancer 15:395
Budwit-Novotny DA, McCarty KS, Cox EB, Soper JT, Mutch DG, Creasman WT et al (1986) Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res 46(10):5419–5425
Mutch DG, Soper JT, Budwit-Novotny DA, Cox EB, Creasman WT, McCarty KS Sr et al (1987) Endometrial adenocarcinoma estrogen receptor content: association of clinicopathologic features with immunohistochemical analysis compared with standard biochemical methods. Am J Obstet Gynecol 157(4 Pt 1):924–931
Chin CC, Li JM, Lee KF, Huang YC, Wang KC, Lai HC et al (2016) Selective beta2-AR blockage suppresses colorectal cancer growth through regulation of EGFR-Akt/ERK1/2 signaling, G1-phase arrest, and apoptosis. J Cell Physiol 231(2):459–472
Wong HP, Yu L, Lam EK, Tai EK, Wu WK, Cho CH (2007) Nicotine promotes colon tumor growth and angiogenesis through beta-adrenergic activation. Toxicol Sci 97(2):279–287
Zhang D, Ma Q, Wang Z, Zhang M, Guo K, Wang F et al (2011) Beta2-adrenoceptor blockage induces G1/S phase arrest and apoptosis in pancreatic cancer cells via Ras/Akt/NFkappaB pathway. Mol Cancer 10:146
Burotto M, Chiou VL, Lee JM, Kohn EC (2014) The MAPK pathway across different malignancies: a new perspective. Cancer 120(22):3446–3456
Munabi NC, England RW, Edwards AK, Kitajewski AA, Tan QK, Weinstein A et al (2016) Propranolol targets hemangioma stem cells via cAMP and mitogen-activated protein kinase regulation. Stem Cells Transl Med 5(1):45–55
Zou HX, Jia J, Zhang WF, Sun ZJ, Zhao YF (2013) Propranolol inhibits endothelial progenitor cell homing: a possible treatment mechanism of infantile hemangioma. Cardiovasc Pathol 22(3):203–210
Xie WY, He RH, Zhang J, He YJ, Wan Z, Zhou CF et al (2019) betablockers inhibit the viability of breast cancer cells by regulating the ERK/COX2 signaling pathway and the drug response is affected by ADRB2 singlenucleotide polymorphisms. Oncol Rep 41(1):341–350
Cremaschi GA, Fisher P, Boege F (1991) Beta-adrenoceptor distribution in murine lymphoid cell lines. Immunopharmacology 22(3):195–206
Lamkin DM, Ho HY, Ong TH, Kawanishi CK, Stoffers VL, Ahlawat N et al (2016) beta-Adrenergic-stimulated macrophages: comprehensive localization in the M1–M2 spectrum. Brain Behav Immun 57:338–346
Bellinger DL, Lorton D (2014) Autonomic regulation of cellular immune function. Auton Neurosci 182:15–41
Riether C, Kavelaars A, Wirth T, Pacheco-Lopez G, Doenlen R, Willemen H et al (2011) Stimulation of beta(2)-adrenergic receptors inhibits calcineurin activity in CD4(+) T cells via PKA-AKAP interaction. Brain Behav Immun 25(1):59–66
Lorton D, Bellinger DL (2015) Molecular mechanisms underlying beta-adrenergic receptor-mediated cross-talk between sympathetic neurons and immune cells. Int J Mol Sci 16(3):5635–5665
Cao M, Huang W, Chen Y, Li G, Liu N, Wu Y et al (2021) Chronic restraint stress promotes the mobilization and recruitment of myeloid-derived suppressor cells through beta-adrenergic-activated CXCL5-CXCR2-Erk signaling cascades. Int J Cancer 149(2):460–472
An J, Feng L, Ren J, Li Y, Li G, Liu C et al (2021) Chronic stress promotes breast carcinoma metastasis by accumulating myeloid-derived suppressor cells through activating beta-adrenergic signaling. Oncoimmunology 10(1):2004659
Acknowledgements
This manuscript is dedicated to the memory of our collaborator Sanjiv Sam Gambhir, whose death inspires us. “and yet, we try”.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82173906); the key project of Ministry of Science and Technology, China (2022YFC2010004); Chinese National Major Project for New Drug Innovation. (No. 2019ZX09201-002-006); the key project of Health Commission of Hunan Province (No.202113010141); Natural Science Foundation of Hunan Province, China (No.2021JJ31063 and No.2021JJ31044). Fundamental Research Funds for the Central Universities of Central South University (No.198101066 and No.208111176).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Prof McLeod is on the board of directors for Vyant Bio. He is one of the founders of Interpares Biomedicine and Clariifi and a consultant to Pharmazam and eviCORE Health Solutions. All other authors declared no competing interests for this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wei Li and Jielin Wan contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, W., Wan, J., Chen, C. et al. Dissecting the role of cell signaling versus CD8+ T cell modulation in propranolol antitumor activity. J Mol Med 100, 1299–1306 (2022). https://doi.org/10.1007/s00109-022-02238-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-022-02238-8