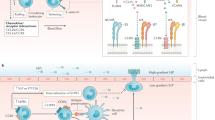
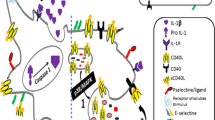
Abstract
Inflammatory bowel disease (IBD) is a devastating disease that is associated with excessive inflammation in the intestinal tract in genetically susceptible individuals and potentially triggered by microbial dysbiosis. This illness markedly predisposes patients to thrombophilia and chronic debility as well as bowel, lymphatic, and liver cancers. Development of new therapies is needed to re-establish long-term immune tolerance in IBD patients without increasing the risk of opportunistic infections and cancer. Aberrant purinergic signaling pathways have been implicated in disordered thromboregulation and immune dysregulation, as noted in the pathogenesis of IBD and other gastrointestinal/hepatic autoimmune diseases. Expression of CD39 on endothelial or immune cells allows for homeostatic integration of hemostasis and immunity, which are disrupted in IBD. Our focus in this review is on novel aspects of the functions of CD39 and related NTPDases in IBD. Regulated CD39 activity allows for scavenging of extracellular nucleotides, the maintenance of P2-receptor integrity and coordination of adenosinergic signaling responses. CD39 together with CD73, serves as an integral component of the immunosuppressive machinery of dendritic cells, myeloid cells, T and B cells. Genetic inheritance and environental factors closely regulate the levels of expression and phosphohydrolytic activity of CD39, both on immune cells and released microparticles. Purinergic mechanisms associated with T regulatory and supressor T helper type 17 cells modulate disease activity in IBD, as can be modeled in experimental colitis. As a recent example, upregulation of CD39 is dependent upon ligation of the aryl hydrocarbon receptor (AHR), as with natural ligands such as bilirubin and 2-(1′ H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE). Decreased expression of CD39 and/or dysfunctional AHR signaling, however, abrogates the protective effects of immunosuppressive AHR ligands. These factors could also serve as biomarkers of disease activity in IBD. Heightened thrombosis, inflammation, and immune disturbances as seen in IBD appear to be associated with aberrant purinergic signaling. Ongoing development of therapeutic strategies augmenting CD39 ectonucleotidase bioactivity via cytokines or AHR ligands offers promise for management of thrombophilia, disordered inflammation, and aberrant immune reactivity in IBD.



Similar content being viewed by others
References
Eltzschig HK, Sitkovsky MV, Robson SC (2012) Purinergic signaling during inflammation. N Engl J Med 367:2322–2333
Longhi MS, Robson SC, Bernstein SH, Serra S, Deaglio S (2013) Biological functions of ecto-enzymes in regulating extracellular adenosine levels in neoplastic and inflammatory disease states. J Mol Med (Berl) 91:165–172
Takenaka MC, Robson S, Quintana FJ (2016) Regulation of the T cell response by CD39. Trends Immunol 37:427–439
Allard B, Longhi MS, Robson SC, Stagg J (2017) The ectonucleotidases CD39 and CD73: novel checkpoint inhibitor targets. Immunol Rev 276:121–144
Luthje J (1989) Origin, metabolism and function of extracellular adenine nucleotides in the blood [published erratum appears in Klin Wochenschr 1989 May 15;67(10):558]. [review]. Klin Wochenschr 67:317–327
Burnstock G, Knight G (2004) Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol 240:31–304
Burnstock G (2002) Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol 22:364–373
Resta R, Yamashita Y, Thompson LF (1998) Ecto-enzyme and signaling functions of lymphocyte CD73. Immunol Rev 161:95–109
Robson SC, Sevigny J, Zimmermann H (2006) The E-NTPDase family of ecto-nucleotidases: structure function relationshhips and pathophysiological significance. Purinergic Signal 2:409–430
Beldi G, Enjyoji K, Wu Y, Miller L, Banz Y, Sun X, Robson SC (2008) The role of purinergic signaling in the liver and in transplantation: effects of extracellular nucleotides on hepatic graft vascular injury, rejection and metabolism. Front Biosci 13:2588–2603
Horenstein AL, Chillemi A, Zaccarello G, Bruzzone S, Quarona V, Zito A, Serra S, Malavasi F (2013) A CD38/CD203a/CD73 ectoenzymatic pathway independent of CD39 drives a novel adenosinergic loop in human T lymphocytes. Oncoimmunology 2:e26246
Cekic C, Linden J (2016) Purinergic regulation of the immune system. Nat Rev Immunol 16:177–192
Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M et al (2007) Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 204:1257–1265
Dwyer KM, Hanidziar D, Putheti P, Hill PA, Pommey S, McRae JL, Winterhalter A, Doherty G, Deaglio S, Koulmanda M et al (2010) Expression of CD39 by human peripheral blood CD4+ CD25+ T cells denotes a regulatory memory phenotype. Am J Transplant 10:2410–2420
Cohen HB, Briggs KT, Marino JP, Ravid K, Robson SC, Mosser DM (2013) TLR stimulation initiates a CD39-based autoregulatory mechanism that limits macrophage inflammatory responses. Blood 122:1935–1945
Longhi MS, Moss A, Bai A, Wu Y, Huang H, Cheifetz A, Quintana FJ, Robson SC (2014) Characterization of human CD39+ Th17 cells with suppressor activity and modulation in inflammatory bowel disease. PLoS One 9:e87956
Jin X, Shepherd RK, Duling BR, Linden J (1997) Inosine binds to A3 adenosine receptors and stimulates mast cell degranulation. J Clin Invest 100:2849–2857
Joos G, Jakim J, Kiss B, Szamosi R, Papp T, Felszeghy S, Saghy T, Nagy G, Szondy Z (2017) Involvement of adenosine A3 receptors in the chemotactic navigation of macrophages towards apoptotic cells. Immunol Lett 183:62–72
Di Virgilio F, Boeynaems JM, Robson SC (2009) Extracellular nucleotides as negative modulators of immunity. Curr Opin Pharmacol 9:507–513
Sun X, Han L, Seth P, Bian S, Li L, Csizmadia E, Junger WG, Schmelzle M, Usheva A, Tapper EB et al (2013) Disordered purinergic signaling and abnormal cellular metabolism are associated with development of liver cancer in Cd39/ENTPD1 null mice. Hepatology 57:205–216
Gao L, Dong L, Whitlock JP Jr (1998) A novel response to dioxin. Induction of ecto-ATPase gene expression. J Biol Chem 273:15358–15365
Gandhi R, Kumar D, Burns EJ, Nadeau M, Dake B, Laroni A, Kozoriz D, Weiner HL, Quintana FJ (2010) Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat Immunol 11:846–853
Mascanfroni ID, Yeste A, Vieira SM, Burns EJ, Patel B, Sloma I, Wu Y, Mayo L, Ben-Hamo R, Efroni S et al (2013) IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat Immunol 14:1054–1063
Hart ML, Gorzolla IC, Schittenhelm J, Robson SC, Eltzschig HK (2010) SP1-dependent induction of CD39 facilitates hepatic ischemic preconditioning. J Immunol 184:4017–4024
Hart ML, Grenz A, Gorzolla IC, Schittenhelm J, Dalton JH, Eltzschig HK (2011) Hypoxia-inducible factor-1alpha-dependent protection from intestinal ischemia/reperfusion injury involves ecto-5′-nucleotidase (CD73) and the A2B adenosine receptor. J Immunol 186:4367–4374
Eltzschig HK, Bonney SK, Eckle T (2013) Attenuating myocardial ischemia by targeting A2B adenosine receptors. Trends Mol Med 19:345–354
Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, Lowes BD, Han J, Borchers CH, Buttrick PM et al (2012) Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med 18:774–782
Perez-Aso M, Feig JL, Mediero A, Cronstein BN (2013) Adenosine A2A receptor and TNF-alpha regulate the circadian machinery of the human monocytic THP-1 cells. Inflammation 36:152–162
Stein AC, Gaetano JN, Jacobs J, Kunnavakkam R, Bissonnette M, Pekow J (2016) Northern latitude but not season is associated with increased rates of hospitalizations related to inflammatory bowel disease: results of a multi-year analysis of a national cohort. PLoS One 11:e0161523
Palmieri O, Mazzoccoli G, Bossa F, Maglietta R, Palumbo O, Ancona N, Corritore G, Latiano T, Martino G, Rubino R et al (2015) Systematic analysis of circadian genes using genome-wide cDNA microarrays in the inflammatory bowel disease transcriptome. Chronobiol Int 32:903–916
Jangi S, Otterbein L, Robson S (2013) The molecular basis for the immunomodulatory activities of unconjugated bilirubin. Int J Biochem Cell Biol 45:2843–2851
Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL (2008) Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 453:65–71
Goettel JA, Gandhi R, Kenison JE, Yeste A, Murugaiyan G, Sambanthamoorthy S, Griffith AE, Patel B, Shouval DS, Weiner HL et al (2016) AHR activation is protective against colitis driven by T cells in humanized mice. Cell Rep 17:1318–1329
Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB (2006) Cutting edge: critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol 177:2765–2769
Frick JS, MacManus CF, Scully M, Glover LE, Eltzschig HK, Colgan SP (2009) Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J Immunol 182:4957–4964
Doherty GA, Bai A, Hanidziar D, Longhi MS, Lawlor GO, Putheti P, Csizmadia E, Nowak M, Cheifetz AS, Moss AC et al (2012) CD73 is a phenotypic marker of effector memory Th17 cells in inflammatory bowel disease. Eur J Immunol 42:3062–3072
Friedman DJ, Kunzli BM, Yi AR, Sevigny J, Berberat PO, Enjyoji K, Csizmadia E, Friess H, Robson SC (2009) From the cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci U S A 106:16788–16793
Orru V, Steri M, Sole G, Sidore C, Virdis F, Dei M, Lai S, Zoledziewska M, Busonero F, Mulas A et al (2013) Genetic variants regulating immune cell levels in health and disease. Cell 155:242–256
Roederer M, Quaye L, Mangino M, Beddall MH, Mahnke Y, Chattopadhyay P, Tosi I, Napolitano L, Terranova Barberio M, Menni C et al (2015) The genetic architecture of the human immune system: a bioresource for autoimmunity and disease pathogenesis. Cell 161:387–403
Mangino M, Roederer M, Beddall MH, Nestle FO, Spector TD (2017) Innate and adaptive immune traits are differentially affected by genetic and environmental factors. Nat Commun 8:13850
Mitsuhashi S, Feldbrugge L, Csizmadia E, Mitsuhashi M, Robson SC, Moss AC (2016) Luminal extracellular vesicles (EVs) in inflammatory bowel disease (IBD) exhibit proinflammatory effects on epithelial cells and macrophages. Inflamm Bowel Dis 22:1587–1595
Jiang ZG, Wu Y, Csizmadia E, Feldbrugge L, Enjyoji K, Tigges J, Toxavidis V, Stephan H, Muller CE, McKnight CJ et al (2014) Characterization of circulating microparticle-associated CD39 family ecto-nucleotidases in human plasma. Purinergic Signal. doi:10.1007/s11302-014-9423-6
Banz Y, Beldi G, Wu Y, Atkinson B, Usheva A, Robson SC (2008) CD39 is incorporated into plasma microparticles where it maintains functional properties and impacts endothelial activation. Br J Haematol 142:627–637
Haller CA, Cui W, Wen J, Robson SC, Chaikof EL (2006) Reconstitution of CD39 in liposomes amplifies nucleoside triphosphate diphosphohydrolase activity and restores thromboregulatory properties. J Vasc Surg 43:816–823
Atarashi K, Tanoue T, Ando M, Kamada N, Nagano Y, Narushima S, Suda W, Imaoka A, Setoyama H, Nagamori T et al (2015) Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell 163:367–380
Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K et al (2008) ATP drives lamina propria T(H)17 cell differentiation. Nature 455:808–812
Baron L, Gombault A, Fanny M, Villeret B, Savigny F, Guillou N, Panek C, Le Bert M, Lagente V, Rassendren F et al (2015) The NLRP3 inflammasome is activated by nanoparticles through ATP, ADP and adenosine. Cell Death Dis 6:e1629
Ouyang X, Ghani A, Malik A, Wilder T, Colegio OR, Flavell RA, Cronstein BN, Mehal WZ (2013) Adenosine is required for sustained inflammasome activation via the A(2)A receptor and the HIF-1alpha pathway. Nat Commun 4:2909
Wang Y, Telesford KM, Ochoa-Reparaz J, Haque-Begum S, Christy M, Kasper EJ, Wang L, Wu Y, Robson SC, Kasper DL et al (2014) An intestinal commensal symbiosis factor controls neuroinflammation via TLR2-mediated CD39 signalling. Nat Commun 5:4432
Sansom FM, Robson SC, Hartland EL (2008) Possible effects of microbial ecto-nucleoside triphosphate diphosphohydrolases on host-pathogen interactions. Microbiol Mol Biol Rev 72:765–781 table of contents
Vaughn BP, Vatanen T, Allegretti JR, Bai A, Xavier RJ, Korzenik J, Gevers D, Ting A, Robson SC, Moss AC (2016) Increased intestinal microbial diversity following fecal microbiota transplant for active Crohn’s disease. Inflamm Bowel Dis 22:2182–2190
Chiaro TR, Soto R, Zac Stephens W, Kubinak JL, Petersen C, Gogokhia L, Bell R, Delgado JC, Cox J, Voth W et al (2017) A member of the gut mycobiota modulates host purine metabolism exacerbating colitis in mice. Sci Transl Med 9
Sivignon A, de Vallee A, Barnich N, Denizot J, Darcha C, Pignede G, Vandekerckove P, Darfeuille-Michaud A (2015) Saccharomyces cerevisiae CNCM I-3856 prevents colitis induced by AIEC bacteria in the transgenic mouse model mimicking Crohn’s disease. Inflamm Bowel Dis 21:276–286
Lavoie EG, Gulbransen BD, Martin-Satue M, Aliagas E, Sharkey KA, Sevigny J (2011) Ectonucleotidases in the digestive system: focus on NTPDase3 localization. Am J Physiol Gastrointest Liver Physiol 300:G608–G620
Kusu T, Kayama H, Kinoshita M, Jeon SG, Ueda Y, Goto Y, Okumura R, Saiga H, Kurakawa T, Ikeda K et al (2013) Ecto-nucleoside triphosphate diphosphohydrolase 7 controls Th17 cell responses through regulation of luminal ATP in the small intestine. J Immunol 190:774–783
Zimmermann H, Zebisch M, Strater N (2012) Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal 8:437–502
Heine P, Braun N, Heilbronn A, Zimmermann H (1999) Functional characterization of rat ecto-ATPase and ecto-ATP diphosphohydrolase after heterologous expression in CHO cells. Eur J Biochem 262:102–107
Shi JD, Kukar T, Wang CY, Li QZ, Cruz PE, Davoodi-Semiromi A, Yang P, Gu Y, Lian W, Wu DH et al (2001) Molecular cloning and characterization of a novel mammalian endo-apyrase (LALP1). J Biol Chem 276:17474–17478
Wink MR, Braganhol E, Tamajusuku AS, Lenz G, Zerbini LF, Libermann TA, Sevigny J, Battastini AM, Robson SC (2006) Nucleoside triphosphate diphosphohydrolase-2 (NTPDase2/CD39L1) is the dominant ectonucleotidase expressed by rat astrocytes. Neuroscience 138:421–432
Belcher SM, Zsarnovszky A, Crawford PA, Hemani H, Spurling L, Kirley TL (2006) Immunolocalization of ecto-nucleoside triphosphate diphosphohydrolase 3 in rat brain: implications for modulation of multiple homeostatic systems including feeding and sleep-wake behaviors. Neuroscience 137:1331–1346
Cardoso AM, Schetinger MR, Correia-de-Sa P, Sevigny J (2015) Impact of ectonucleotidases in autonomic nervous functions. Auton Neurosci 191:25–38
Braun N, Sevigny J, Robson SC, Hammer K, Hanani M, Zimmermann H (2004) Association of the ecto-ATPase NTPDase2 with glial cells of the peripheral nervous system. Glia 45:124–132
Di Giovangiulio M, Verheijden S, Bosmans G, Stakenborg N, Boeckxstaens GE, Matteoli G (2015) The neuromodulation of the intestinal immune system and its relevance in inflammatory bowel disease. Front Immunol 6:590
Gabanyi I, Muller PA, Feighery L, Oliveira TY, Costa-Pinto FA, Mucida D (2016) Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell 164:378–391
Gallego D, Gil V, Martinez-Cutillas M, Mane N, Martin MT, Jimenez M (2012) Purinergic neuromuscular transmission is absent in the colon of P2Y(1) knocked out mice. J Physiol 590:1943–1956
Galligan JJ (2002) Ligand-gated ion channels in the enteric nervous system. Neurogastroenterol Motil 14:611–623
Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, Muruve DA, McKay DM, Beck PL, Mawe GM et al (2012) Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med 18:600–604
Gombault A, Baron L, Couillin I (2012) ATP release and purinergic signaling in NLRP3 inflammasome activation. Front Immunol 3:414
Fields RD, Stevens B (2000) ATP: an extracellular signaling molecule between neurons and glia. Trends Neurosci 23:625–633
Ruhl A (2005) Glial cells in the gut. Neurogastroenterol Motil 17:777–790
Brown IA, McClain JL, Watson RE, Patel BA, Gulbransen BD (2016) Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin-43 and nitric oxide. Cell Mol Gastroenterol Hepatol 2:77–91
Eltzschig HK, Bratton DL, Colgan SP (2014) Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat Rev Drug Discov 13:852–869
Ochoa-Cortes F, Linan-Rico A, Jacobson KA, Christofi FL (2014) Potential for developing purinergic drugs for gastrointestinal diseases. Inflamm Bowel Dis 20:1259–1287
Acknowledgements
The work summarized in this review article was supported by the National Institute of Health grants to SCR and MSL; R01 HL094400; R01 DK108894; P01HL107152, and P01 HL087203 as well as the generosity of the family of Jane O. Siegel; as well as a Clinical Research Award from the American Gastroenterology Association and the Alan Holfman Clinical and Translational Research Award from the American Association for the Study of Liver Disease to ZGJ. We thank Eliza Robson for the graphic design for figures.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Longhi, M.S., Moss, A., Jiang, Z.G. et al. Purinergic signaling during intestinal inflammation. J Mol Med 95, 915–925 (2017). https://doi.org/10.1007/s00109-017-1545-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-017-1545-1