Abstract
The importance of creating eco-friendly and health-conscious materials has become paramount in striving to attain long-term development goals. For the past decades, constant efforts have been made to tackle the issue of formaldehyde release from wood-based panels which, to date, are still mainly produced from unsustainable synthetic adhesives. In the pursuit of sustainable and environmentally responsible adhesive solutions for the wood industry, sodium bisulfate, sodium bisulfite, and sodium nitrite were used under different heat treatment conditions as crosslinkers for canola protein-based bio-adhesive formulations. The developed adhesive formulations showed outstanding mechanical properties, with a viscosity below 4000 mPa/s despite the relatively high solid content, as well as excellent bonding performances. The one-layer particleboards bonded with the canola-based adhesive demonstrated outstanding mechanical properties, with the internal bonding and the bending strength values surpassing 0.60 N/mm2 and 10 N/mm2, respectively. Notably, the sodium nitrite-crosslinked variants exhibited significantly superior performance compared to the UF-bonded control boards. Longer incubation times generally improve bonding strength, with sodium nitrite showing the most pronounced effects. The results of this research showcase not only the possibility of developing a plant protein-based wood adhesive with high solid content, but also the potential superiority of canola protein-based wood adhesives when compared to conventional, synthetic counterparts. These findings offer valuable insights for optimizing bio-based adhesives in wood composite manufacturing, highlighting sodium nitrite as a promising crosslinker for enhancing the adhesive’s performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The wood adhesive industry currently relies heavily on formaldehyde-based adhesives. While these adhesives are cost-effective, their production relies on non-renewable resources, and formaldehyde is recognized as a carcinogen (Adhikari et al. 2017). With the depletion of non-renewable resources becoming more acute and increasing concerns about environmental protection (Xiao et al. 2023), there is a need to transition away from conventional adhesives to achieve sustainable development goals. In recent decades, driven by societal awareness and concerns about indoor air quality, health, and the environment, there has been a shift towards developing environmentally friendly and sustainable alternatives to conventional formaldehyde-based resins (Cárdenas-Oscanoa et al., 2024). This shift is further motivated by the desire to reduce costs using low-cost, abundant materials such as various lignin types as by-products, and to decrease formaldehyde emissions (Dunky 2021). From the numerous candidates studied so far, plant protein-based adhesives have emerged as promising alternatives, offering biodegradability, renewability, and low toxicity. Among plant proteins, soy has received the most attention to date (Chen et al. 2023; Li et al. 2022a; Li et al. 2022b; Vnučec et al. 2017), due to its dominance in the market (Adhikari et al. 2017; He 2017; Lusas 2000; Wool and Sun 2005). Despite the extensive attention given to soy, there is a growing understanding of the importance of diversifying protein sources for bio adhesives (Barzegar et al. 2020, 2022; Dunky 2021; Frihart 2023; Kallakas et al. 2024; Solt et al. 2019) to ensure the long-term production and supply of protein-based bio adhesives. This diversification effort is essential for enhancing the resilience of the bio adhesive industry and reducing dependence on a single protein source.
Canola, a cultivar of rapeseed developed in Canada in 1976, now refers to three species of the genus Brassica that produce oil with low level of erucic acid (less than 2%), and less than 30 µmol/g meal of total glucosinolates: Brassica rapa, Brassica napus and Brassica juncea (Bell 1995. Although canola is the second most abundant oilseed after soy, defatted canola has largely been overlooked as an industrial material (Adhikari et al. 2017; Li et al. 2017). Primarily cultivated for its oil, the processing of canola generates a significant amount of by-products (Bandara et al. 2017). Despite its availability, the use of canola meal has mainly been limited to low-value animal feed or as fertilizer (Manamperi et al. 2010; Wang et al. 2014), due to the presence of glucosinolates, erucic acid, phytates, and phenolics, which render it unsuitable for human consumption (Hale 2013). Exploring high-value uses of canola protein products, such as adhesives for wood composites, could significantly enhance the economics of the canola oil industry (Manamperi et al. 2010). Canola protein is stored in two main components: napin and cruciferin, which constitute 20% and 60% of the total protein in mature seeds, respectively (Höglund et al. 1992). They are characterized by complex protein compositions (Li et al. 2012). High molecular weight cruciferins are neutral proteins, which belong to the albumin storage protein type. The cruciferin has a hexamer structure like soy glycinin protein (Bérot et al. 2005). Both covalent (disulfide bonds) and non-covalent bonds are present in the cruciferin protein structure (Wu and Muir 2008), with the covalent bonds dominating in the protein structure. Napins are low-molecular weight basic proteins made of two disulphide linked polypeptide chains stabilized by disulfate bonds (Ericson et al. 1986; Gehrig and Biemann 1996; Schwenke et al. 1981).
Despite its potential suitability for the development of bio-adhesives (Tene Tayo et al. 2022), canola protein has received less attention compared to other plant proteins such as soy and wheat. The relatively low protein content in canola flour may explain why most of the research has focused on isolated proteins rather than the meal or flour (Li et al. 2012, 2017; Manamperi et al. 2011; Tzeng et al. 1990). However, it is important to note that each oilseed has its own unique protein structures and properties, and cohesive strength may not necessarily correlate with protein content (Barzegar et al. 2020). Combining canola protein with other protein sources and employing denaturation and crosslinking processes may enhance the bonding properties of canola-based adhesives.
Since plant protein-based and plant flour-based adhesives typically do not provide sufficient wet bond strength on their own, the inclusion of additional co-reactants or crosslinkers is necessary (Adhikari et al. 2017, 2017; Barzegar et al. 2020; Bjorksten 1951; Frihart and Lorenz 2019, 2018; Hettiarachchy and Kalapathy 1998; Shi et al. 2017; Vnučec et al. 2017). Although such an approach helps reduce the amount of formaldehyde release, it does not fully resolve the issue of sustainability. Therefore, the effect of more environmentally friendly crosslinkers needs to be investigated. A previous study revealed that canola meal adhesive could be used to produce medium density fiberboards with satisfactory mechanical properties (Tene Tayo et al. 2022). Although the direct use of the meal helps to avoid expensive and low-yield protein isolation process, the low protein content of canola meal, as well as the low solid content of the adhesive make it difficult for application in particleboards. The use of protein isolate with high protein concentration might then help overcome this limitation.
Gelatine, a protein derived from collagen, consists of 18 varieties of complex amino acids, with glycine, proline, and hydroxyproline being the majority (Sultana et al. 2018). Chemically, gelatine is composed of 25.2% oxygen, 6.8% hydrogen, 50.5% carbon, and 17% nitrogen, and it comprises a mixture of single and double unfolded chains with hydrophilic characteristics (Nur Hanani et al. 2014). The chemical structure of gelatine includes various polypeptide chains such as α-chains (single chains), β-chains (two α-chains covalently crosslinked), and γ-chains (three covalently crosslinked α-chains), with molar masses of approximately 90,000, 180,000, and 300,000 g/mol, respectively (Mariod and Fadul 2013). Urea, a denaturing osmolyte, is widely used to assess protein stability and the effects of mutations on stability, as well as protein unfolding (Pace 1986). Urea may directly exert its effect by binding to the protein and competing with native interactions, thereby actively participating in the unfolding process (Das and Mukhopadhyay 2009; Hua et al. 2008; Klimov et al. 2004). As a denaturing agent, urea disrupts the protein’s structure and exposes functional groups, thereby enhancing the properties of the protein adhesive (Chen et al. 2019, 2021; Zhang and Hua 2007). When incorporated into adhesive formulations, gelatine and urea contribute to enhancing adhesion strength, water resistance, and mechanical properties. In the present study, the effect of sodium bisulfate, sodium bisulfite, and sodium nitrite as crosslinkers, coupled with heat treatment, was investigated on gelatine-reinforced canola protein adhesive formulations, with the aim of improving the bonding properties of the wood adhesive.
2 Materials and methods
2.1 Materials
The canola protein (Puratein® G) with a protein concentration of 90% was purchased from Merit Functional Foods, Winnipeg, Canada. Sodium dodecyl sulfate (SDS), urea, sodium bisulfate (92%), sodium bisulfite (98%), sodium chloride, and sodium nitrite (99%) were sourced from VWR International in Darmstadt, Hesse, Germany. The gelatine (180 Bloom) was obtained from Carl Roth GmbH + Co KG in Karlsruhe, Germany. The hydrophobic agent, SASOL Wax Pro 18 A, with a solids content of 60%, was acquired from SASOL Wax GmbH in Hamburg, Germany. This is a water repellent which helps to improve the water resistance of particleboard. The wood chips were supplied by Pfleiderer in Arnsberg, North Rhine-Westphalia, Germany. The particle size distribution of the wood particle material is detailed in Table 1.
2.2 Methods
2.2.1 Adhesive preparation
The various formulations were prepared by initially dissolving the required quantities of gelatine, SDS, urea, and water. Heat could be applied to facilitate this process. Subsequently, sodium chloride and either sodium bisulfate, sodium bisulfite, or sodium nitrite were added in specified proportions (as outlined in Table 2). The majority of plant proteins consists predominantly of salt-soluble globulins and water-soluble albumins, typically in a ratio of approximately 70–20%, varying slightly based on the origin of the plant proteins (Lu et al. 2020; Singhal et al. 2016). Sodium chloride was therefore employed to enhance protein solubility, thereby contributing to the solid content of the adhesive. Additionally, sodium bisulfate, sodium bisulfite, and sodium nitrite were utilized as crosslinkers to enhance adhesive performance. Urea, on the other hand, was added to modify the protein structure (Lifson and Roig 1961; Nick Pace and Scholtz 1998), while improving its interaction with other components of the adhesive mixture. The canola protein isolate (CPI) was then gradually introduced while stirring with an RW 20 laboratory stirrer from IKA®-Werke GmbH & Co. KG, rotating at 10,000 rpm. To ensure proper denaturation of the canola protein and expose more active groups within the protein chain, the pH of the slurry was adjusted to 11 ± 0.2. From preliminary experiments investigating the pH effect, it was determined that increasing the pH of the protein-based binder enhances bonding properties. However, extreme denaturation of the protein (pH above 12) adversely impacts the stability of the binder, reducing its quality and shelf life (Tene Tayo et al. 2022). The effect of heat treatment was evaluated by incubating the slurries at 60 °C for 30, 45, and 60 min using the IKA® LR 1000 modular laboratory reactor from IKA Werke GmbH & Co. KG, rotating at 130 rpm. Twelve different adhesive variants were prepared based on three chemicals (sodium bisulfate, sodium bisulfite, and sodium nitrite) and four different incubation times (0, 30, 45, 60 min). The different adhesives had a solid content of 55%. The reaction time is known to be crucial for an optimal crosslinking of protein-based adhesives (Zeng et al. 2023). Therefore, they were labelled (Bisulfate-T, Bisulfite-T, and Nitrite-T, with T representing the incubation time) and stored in glass containers for one week at room temperature before being utilized in the production of one-layer particleboards.
2.2.2 Description of the prepared bio-adhesive
Figure 1 shows the physical behaviour of the adhesives within the first days of preparation. During incubation, an increase in the volume was noticed, probably due to the increase of air bubbles in the mixture during the process. After resting for 1 to 2 days, a foaming process at the surface was observed. It is known that foaming occurs when air is trapped in the protein slurry, forming more or less stable air pockets in the mixture. The process is enhanced by the hydrophobic (water repelling molecules) and the hydrophilic (water attracting molecules) nature of the protein molecule. While the hydrophilic molecules are attracted by water, the hydrophobic molecules are repelled and they link to the air bubbles, eventually making the foam. Simply stirring the mixture made the foam layer at the surface to disappear. However, the presence of the air bubbles made it difficult to measure the viscosity of the adhesives. Therefore, the air bubbles were taken out of the slurries using a vacuum oven from Memmert GmbH + Co. KG, Schwabach, Bavaria. Afterwards, a smooth and homogeneous fluid was obtained and no further foaming was observed.
2.2.3 Determination of the dynamic viscosity
The determination of the dynamic viscosity in mPa/s was determined based on DIN EN 12092 (2002). For this purpose, the test method using the rotational viscometer method was applied. The spindle viscometer used was the ROTAVISC lo-vi Complete from IKA Werke GmbH & Co. KG. Initially, a predetermined volume of binder was dispensed into a beaker, adjusted according to the chosen spindle size, which was determined based on the viscosity of the sample. Subsequently, the device underwent calibration, and the rotational speed of the spindle was set. The binder was then brought to a uniform temperature of 25 °C, using a temperature-controlled water bath. Following this, the spindle of the instrument was submerged into the tempered binder up to the notch, initiating the measurement process. The measurements were iterated until the collected data exhibited less than a 3% variance. The test was repeated five times post-adjustment, and subsequently, the average dynamic viscosity was calculated and reported in mPa/s.
2.2.4 Production of one-layer particleboards
The one-layer particleboards were produced on pilot scale in the biotechnikum laboratory of the Burckhardt Institute, university of Goettingen, Germany. Prior to the production, the wood chips were dried to a moisture content of about 2% using a universal oven from the company Memmert (model UN45). The necessary amount of wood chips was weighed, as well as the amount of resin needed. The hydrophobic agent was mixed with the adhesive prior to application. A resin load of 10% based on the oven-dried wood material was applied onto the wood particles in a rotative blending drum using the air-pressure atomizer nozzle (Düsen-Schlick GmbH, Coburg, Germany). A reference variant was made using a conventional UF 345 resin with a solid content of 68%. The same resin load was applied to the reference boards as for the protein-based adhesive bonded ones (10%). The boards were preformed using a 0.3 m x 0.485 m mat former and pre-pressed. The target board density was 640 kg/m³ at 15 mm thickness. The hot-pressing was performed using a computer‐controlled laboratory‐scale hydraulic single‐opening hot‐press (Siempelkamp Hydraulic Lab Press A 308/1988). The production parameters are shown in Table 3. For each treatment, four boards (repetitions) were produced. After production, the boards were conditioned at room temperature for 24 h. They were trimmed to avoid edge effects and sanded on both sides by using a wide‐belt sanding machine (Felder type FW 950 C from Felder Group, Hall In Tirol, Austria) before being tested.
2.2.5 Water extractive content and pH measurement
Cold-water extraction was made on the produced panels in order to not only compare the amount of extractive contained in the particleboards, but also more importantly to assess variation in pH between the protein-bonded and the UF-bonded boards. For this purpose, some pieces of the particleboards were pulverized using a speed rotor mill (Fritsch GmbH, Idar-Oberstein, Germany) to obtain a powder (< 80 μm) for the experiment.
2.2.6 SEM
Cross-section morphology of samples was observed with a LEO Supra-35 high-resolution field emission SEM (Carl Zeiss AG, Germany) using a 5 kV acceleration voltage and an average magnification of 80X. Given a limited capacity, only the particleboards bonded with unincubated and the 60 min incubated adhesive variants were scanned. Samples were previously sputtered in a SC7620 Mini Sputter-Coater, Qorum. The optical microscope pictures were taken from the cured adhesive after drying at 105 °C overnight.
2.2.7 Testing the mechanical properties of the produced particleboards
The produced particleboards were then tested for their mechanical properties. The internal bonding strength (IB) was tested following EN 319 (1993), while the bending strength (BS) and the modulus of elasticity (MOE) were tested in accordance with EN 312 (1993) and EN 310 (1993) respectively, using the universal testing machine ZWICK/ROELL (type 10). Five test samples of 50 by 400 mm were cut off each of the boards for the BS and the MOE test. Five 50 by 50 mm test pieces were also prepared from each produced board for the IB test. The chosen samples were those with the density closest to the board’s target density (640 kg/m3).
2.2.8 Data analysis
The data analysis was performed for the mechanical properties of particleboards. The experiment was conducted through a completely randomized design with two factors: crosslinker (with three levels: NaSO4, NaSO3, NaNO2) and incubation time (with four levels: 0, 30, 45 60 min). An analysis of variance (ANOVA) (ρ < 0.05) was conducted to test whether either or both of the factors significantly influence the mechanical properties of the particleboards. The data analysis was performed with the R version 4.3.1 from R Development Core Team.
3 Results and discussion
3.1 Cold-water extractives and pH
The binding reaction between the adhesive and wood, much like many other chemical processes, is notably influenced by variations in pH. The pH value of wood, or more precisely of the aqueous solution within humid wood, is very important for various ranges of its utilization and might influence the adhesive power of glues (Feldman, 1985). The pH and the buffer capacity of wood materials are innate properties of the wood that cannot be controlled. Most wood species have a pH value ranging between 3 and 6. It is reported that either high or low pH of an adhesive could influence its bond durability and performance of the engineered wood product (Zhang et al. 2010). Because a low pH affects predominantly the holocellulose in the wood structure, a low pH value may have a more detrimental effect on wood strength than a high pH (Stamm 1964). There are also concerns that not only the pH, but also the buffering capacity may significantly impact the adhesion performance, thereby affecting the mechanical properties of the wood and impact the service life of the glued-wood product (Policardi and Thebault 2020). Attention should therefore be put on to the pH of wood in connection with fibre- and particleboard production (Kehr and Schilling 1965; Sandermann and Rothkamm 1959), as well as on the capacity on the wood to neutralize either the acidity or the alkalinity of the binder without the properties of the wood product being affected. Although the denaturation process of plant protein for better bonding effect has been realized both in acidic and alkaline media, most plant protein-based wood adhesives have proven to be more efficient at high pH. This is because the structure of the protein is best modified and the carboxylic and amine groups of the protein chain are made available. Fig. 2 presents the values of the cold-water extractives and the pH of the aqueous solution obtained from the produced protein-bonded particleboards. The pH of spruce wood for cold water extractives is 4.9 (Feldman, 1985), which is lower than what was obtained in the present study for the wood particles used (6.33). The reason of this difference in pH is due to the fact that the particles used in the present study contain recycled wood. The natural pH has then been affected by previous processing operations. The pH values of the different protein-bonded boards were similar to each other (6.78, 6.99 and 6.89 for Bisulfate-T, Bisulfite-T and Nitrite-T variants respectively), while being slightly higher than that of the UF-bonded boards. Knowing that the pH of the binders was around 11, these values show a good buffering capacity of the wood chips used, proving that using a binder with high pH value poses no issue for the wood product, as far as the raw wood material has a good ability of neutralizing the effect. This assertion aligns with findings from studies on synthetic binders like phenol formaldehyde (Zhang et al. 2010) or pMDI (Pizzi 2016) which exhibits pH ranging from low (6–8 for pMDI) to high (about 13 for PF resin) alkaline. Among the particleboard variants, the cold-water extractive amounts followed a similar pattern to pH, with the highest observed in the Bisulfite-T variants (6.41%), followed by the Nitrite-T and the Bisulfate-T variants (5.91% and 5.56%, respectively). UF-bonded and native particles exhibited lower extractive amounts (3.87% and 3.48%, respectively). The higher extractives in protein-bonded boards compared to other variants are attributed to water-soluble compounds present in the natural binder (Ostendorf et al. 2021a). Surprisingly, a higher amount of cold-water extractives was obtained from the protein binder dried overnight at 105° C. The 60% extractive content demonstrates a high vulnerability of the adhesive to hydrolysis, suggesting the dominance of hydrogen bonds over covalent bonds in the adhesive matrix. The difference between the amount of extractives from the particleboard and that from the cured binder might be attributed to some chemical interaction occurring between the wood and the binder during the hot-pressing which might affect the extractability of some component.
3.2 Rheological properties of the adhesive formulations
Three primary factors determine the adhesion strength: (i) physicochemical interactions, influenced by the extent and nature of surface treatment, (ii) surface roughness, and (iii) the quantity of chemical bonds formed per unit area between the resin and the substrate (Nardin and Ward 1987). Achieving a sufficiently strong bond requires proper penetration of the adhesive depth into the wood surface, enabling intimate molecular contact with the substrate (Cheng and Sun 2006). The viscosity of a wood adhesive plays a crucial role in this process as well, affecting the penetration behaviour of the adhesive into the wood surface (Scheikl and Dunky 1998). Striking a balance in viscosity is essential for facilitating wood blending, ensuring better distribution, and promoting adhesive penetration. While a low viscosity leads to excessive penetration, hindering the formation of an adhesive layer between wood particles, very high viscosity makes it difficult to spray the adhesive and results in uneven distribution, thereby affecting panel bonding strength. The addition of the salts significantly affected the viscosity of the prepared adhesives, with S.bisulfite yielding the lowest values, followed by S. bisulfate and then S. nitrite (Fig. 3). The heat treatment was also found to highly impact the adhesive viscosity. While the untreated variants had values of 3785 mPa/s, 2299 mPa/s and 5327 mPa/s for Bisulfate-T, Bisulfite-T and Nitrite-T respectively, the viscosity of their corresponding 30 min treated variants went down to 2900 mPa/s, 1947 mPa/s and 2581 mPa/s. The highest impact was observed with S. nitrite, with a reduction of more than 50% of the viscosity after 30 min of heat treatment. Surprisingly, prolonged treatment caused the viscosity to increase again after 30 min, with values increasing with increasing incubation time. The effect of incubation time was found to be more detrimental to the S. bisulfite variant, with the viscosity of one hour-treated variant being higher than that of the untreated. These results suggest that a 30 min treatment will be optimal for a good viscosity. Cheng and Sun (2006) described the viscosity of soybean protein adhesive as a result of intermolecular interactions between the protein molecules. Addition of NaSO4 to the NaOH-modified soybean protein adhesive helped reduce the viscosity, with the effect increasing with increasing concentration of NaSO4. The same effect was observed during the preparation of canola protein adhesive, which was furthermore enhanced by the heat treatment, which helped improve the interaction between molecules. It was reported by Alavi et al. (2021) that heating under alkaline conditions significantly affects the protein, improving the solubility, as well as its functional properties. The solid content of plant protein adhesives (e.g., soy-based adhesives) usually ranges from 32 to 36% (Huang and Li 2008; Lorenz et al. 2007). Despite such a relatively low solid content as compared to the conventional UF resin (about 66%), their viscosity usually remains high, with values above 6000 mPa/s (Eslah et al. 2016). Therefore, despite a higher solid content (55%), low viscosity of the adhesive formulation achieved in the present study is attributed to the reduced electrostatic interaction between the protein molecules.
3.3 SEM
The microscopic analysis of the particleboard samples revealed crucial insights into the bonding between particles and binder, which plays a pivotal role in determining their mechanical and technological properties. A key indicator of satisfactory bonding was the absence of voids, signifying a robust joint between particles and binder. The presence of voids in any material can significantly impact its physical and mechanical characteristics. Upon examination, optical microscopic images of the dried adhesive displayed a similar structural pattern for both bisulfate and nitrite adhesives, characterized by numerous circular or oval spaces, likely formed due to the presence of air bubbles. Interestingly, the bisulfite adhesive didn’t show a notable absence of such bubbles or spaces. This distinction between bisulfite and the other adhesives may explain the comparatively lower mechanical properties observed in particleboards produced with bisulfite adhesive. Moreover, a discernible difference was noted in the crystallization of the adhesive, with the nitrite adhesive exhibiting a shinier surface, indicative of better crystallization, and correlating with higher mechanical property values.
Furthermore, microscopic examination revealed a heterogeneous mixture of spruce and pine wood particles and cylindrical fibers ranging from 0.25 to 3 mm in length. While the wood particles were integrated with the binder material, the fibers and particle positions appeared randomized. However, during the pressing process, the fibers tended to align parallel to the plane, while the binding resin formed a cell-like structure that filled the empty spaces between the wood particles. This resinous matrix was distinguishable from the wood particles due to its larger size, solid shape appearance, and random arrangement. Conversely, the fibers exhibited an elongated and sinusoidal form, with discernible anatomical elements such as intervascular pits and, in some cases, the fiber cell wall and lumen.
Comparative analysis of scanning electron microscope (SEM) images of samples treated with different binding agents revealed distinct fracture morphologies. The use of nitrite-containing adhesive resulted in a less distinct fracture morphology compared to bisulfate and bisulfite adhesives (Fig. 4). Additionally, the distribution of dried nitrite adhesive differed significantly from that of bisulfate adhesive. Bisulfate-treated samples displayed single and cross-field holes in the particle board core, contributing to porous properties that may affect mechanical performance. Notably, bisulfite-treated samples exhibited a more heterogeneous surface, with incubation time negatively impacting internal bonding values by up to 18%. The internal bonding, a critical mechanical property of the wood-based composite, is highly sensitive to particle-binder distribution (Niemz et al. 2023). Samples treated with nitrite adhesive and subjected to a 60-minute incubation period demonstrated optimal fiber embedding and a homogeneous surface, resulting in superior mechanical performance in tests such as internal bonding, bending strength, and elasticity modulus.
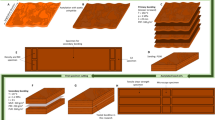
Cross section SEM images of the produced particleboards bonded with adhesive formulations (A) Bisulfate-0 min; (B) Bisulfate-60 min; D) Bisulfite-0 min; E) Bisulfite-60 min; G) Nitrite-0 min; H) Nitrite-60 min, and optical microscopic images of Bisulfate (C), Bisulfite (F) and Nitrite (I) dried adhesives
3.4 Physical and mechanical properties of the particleboards
The physical properties of particleboards, such as thickness and density, are important indicators of their mechanical properties and suitability for various applications. For instance, while the thickness determines the dimensional stability and the load-bearing capacity, the density affects the resistance to mechanical stress and moisture absorption. The mean values of the thickness and the density of the different board variant are presented in Table 4. The average thickness ranged from 14.3 mm to 14.6 mm. A slight variation in thickness was observed among different resin types and incubation time. While Nitrite-T tended to be slightly thicker (with a mean value of 14.43 mm), Bisulfate-T and Bisulfite-T particleboards variants exhibited similar thickness values (14.38 mm and 14.39 mm respectively). Surprisingly, a high thickness was observed from the boards bonded with the unincubated binders in comparison to their incubated counterparts. These differences may be attributed to the variation in resin composition due to the incubation process, as well as to the curing process of the resin (spring back effect due to improper curing of the adhesive). The density values ranged from approximately 628 kg/m3 to 649 kg/m3. Irrespective of the incubation time the different binder types exhibited the same density (638 kg/m3). However, just like in the case of the thickness, variations within the groups were observed.
The internal bonding (IB), as well as the bending strength (BS) and the E-modulus are critical parameters affecting the quality and performance of particleboards. The performance of the adhesive system used in the production determines the strength and the stability of the board. In this study, the effect of the crosslinking agent and that of the incubation time were investigated to understand their effect on the performance of the developed protein adhesive. The overall effect of the different crosslinkers (irrespective of the heat treatment) on the mechanical properties of the particleboards is shown in Fig. 5.
The result of the internal bonding (IB) test revealed significant variations in the bonding property across different variants and incubation time, as the recorded IB values strength ranged from as low as 0.15 N/mm2 to as high as 0.89 N/mm2. Among the crosslinkers, sodium nitrite consistently exhibited higher IB strength compared to sodium bisulfate and sodium bisulfite. As shown by the pairwise comparisons, the nitrite-crosslinked variants consistently demonstrated significantly higher IB strength compared to both bisulfate and bisulfite variants. The high IB value achieved by the nitrite-crosslinked variant was expected due to the chemical reactivity of nitrite ions with protein. Nitrite is known to react with proteins, modifying various amino acids and forming diazeniumdiolates. These diazeniumdiolates can then react with nucleophiles, including thiol (-SH) groups in proteins, as well as with amines and amino groups, forming nitroso compounds. These reactions improve the cross-linking effect, connecting different protein molecules through covalent bonds, contributing to the adhesive’s performance. A study by Woolford et al. (1976) demonstrated that added nitrite to bovine albumin solutions was largely lost within a week, with a significant portion chemically bonded to the protein. This highlights the importance of the one-week timeframe between the production of the binders and testing. Additionally, a research conducted by (Feng et al. 2016) on the influence of sodium nitrite on protein oxidation and nitrisation revealed the antioxidant effect of nitrite on proteins, resulting in decreased sulfhydryls and increased disulfide bonds, as well as improved protein nitrosation. In comparison to bisulfate and bisulfite-treated adhesives, nitrite-crosslinked adhesives consistently outperformed in terms of IB values. While the nitrite-crosslinked variants achieved an average IB of 0.58 N/mm2 irrespective of the heating treatment, the two other variants remained at 0.42 N/mm2 and 0.32 N/mm2 respectively. More interesting is that the nitrite-treated variant could achieve the same IB value as the reference UF 345 (see Table 5).
The bending strength (BS) of the produced particleboards was as well impacted by the crosslinker, with sodium nitrite consistently performing better than sodium bisulfate and sodium bisulfite. The nitrite variant had a BS of 10.24 N/mm2, followed by bisulfate (9.52 N/mm2), UF 345 (9.15 N/mm2) and bisulfite (9.09 N/mm2). Here again, the nitrite-treated variant overcame the reference UF 345 variant. This suggests that nitrite-based adhesives impart greater structural strength to particleboards, possibly due to enhanced bonding with the wood substrate.
Results of the modulus of elasticity show that stiffness of the particleboards bonded with the nitrite-crosslinked adhesive variant was significantly superior to that of both bisulfate and bisulfite-crosslinked, but lower than that of the UF 345-bonded variant. Nevertheless, all four particleboard types demonstrated a stiffness higher than the EN requirement for the particleboards designed for interior use in dry conditions.
Overall, it was observed that IB (Fig. 6), BS (Fig. 7) and the MOE (Fig. 8) were improved with increasing heat treatment time for the Bisulfite-T and the Nitrite-T variants.
The incubation time significantly affected IB strength of the particleboards, with longer exposure to heat treatment generally improving the bonding performance of the adhesive. The interaction effect between crosslinker type and incubation time was also found to affect the mechanical properties of the particleboards. Statistically, the results reveal that certain combinations of crosslinkers and incubation times resulted in synergistic effects, leading to higher IB values than would be expected based on the individual effects of each factor alone. For instance, the nitrite-crosslinked variants exhibited the highest IB strength, particularly after 60 min of incubation, indicating a strong positive interaction between nitrite and prolonged exposure time. The heat treatment significantly improved the IB of Nitrite-T variants (P < 0.001), with values steadily increasing from 0.50 ± 0.06 N/mm2 for the unincubated variant to 0.67 ± 0.05 N/mm2 after 60 min. After 30 min of incubation, the bonding strength of the Nitrite-T variants matched that of the UF-bonded boards. Further heating significantly increased IB. Thermal incubation promoted efficient reactions between nitrite and proteins. With increased mobility of protein molecules and nitrite ions, contact and interaction between adhesive components were further facilitated, leading to strong bond formation. The combination of these factors resulted in IB values significantly superior to that of UF-bonded boards. With IB values exceeding 0.6 N/mm2, the nitrite-crosslinked adhesives could potentially be used for P4 particleboard production, provided thickness swelling requirements are met. In contrast, heat treatment negatively affected the bonding performance of bisulfate-treated adhesive formulations. IB decreased from 0.47 ± 0.06 N/mm2 for the unincubated variant to 0.39 ± 0.03 N/mm2 after 60 min of heating. The highly alkaline environment initially promoted deprotonation of thiol groups, facilitating disulfide bond formation. Bisulfate crosslinking typically involves the formation of ester bonds between the carboxyl groups of amino acids in the adhesive proteins and the hydroxyl groups of bisulfate ions. These bonds are susceptible to hydrolysis under high-temperature conditions, leading to the breakdown of the adhesive matrix, and subsequently to a decrease in bonding strength. Therefore, prolonged heating might have adverse effects, such as protein structure alteration or over-cross-linking, leading to rigid or brittle adhesives and reduced performance. SEM results confirmed better crystallization of the cured binder for Nitrite-T variants, corroborating the IB values obtained. Nevertheless, although the incubation time had a negative impact on the internal bonding (IB) of the bisulfate-treated variants, both the bending strength (BS) and modulus of elasticity (MOE) showed improvement. Among the Bisulfite-T variants, the recorded IB value ranged from 0.28 ± 0.04 N/mm2 for the unincubated variant to 0.41 ± 0.04 N/mm2 after 60 min of heat treatment. Compared to the untreated variant, 30 min of treatment time resulted in no significant change in bonding strength. However, further heat treatment led to an increase, with values reaching 0.34 ± 0.03 N/mm2 after 45 min and 0.41 ± 0.04 N/mm2 after 60 min. (see Table 6). The highly alkaline environment of sodium bisulfite likely promoted deprotonation of thiol groups in the protein and facilitated the formation of disulfide bonds in the adhesive. Prolonged heating in alkaline conditions may have further facilitated cross-linking or strengthening of disulfide bonds. Heat-induced protein denaturation, coupled with bisulfite presence, exposed more disulfide bonds in the protein chain, providing a stable environment for longer reactions and potentially enhancing adhesive performance. Despite the improved adhesive performance with heating, IB values of the bisulfite-treated variants remained lower than that of the UF-bonded reference boards.
The BS and the E-Modulus were minimally affected by adhesive incubation. Heat treatment time did not affect BS performance of bisulfate- and nitrite-treated adhesives. However, 60-minute treatment significantly improved BS for bisulfite-treated variants. Overall, except for unincubated and 30-minute incubated Bisulfite-T variants, the recorded BS values for bio adhesive formulations were mostly superior to those of UF-bonded boards.
4 Conclusion
The dependency of the wood industry on formaldehyde-based adhesives has become an ever-greater issue in view of the formaldehyde emissions, as well as their non-renewability. In an attempt to develop alternative solutions, the current study was designed to highlight the potential of canola protein for developing competitive wood adhesives suitable for manufacturing P2 grade particleboards. With significantly higher solid content compared to most existing plant protein-based adhesives, this adhesive formulation represents a notable advancement in the field of bio-based adhesives for wood-based panels. The statistical analysis uncovered significant (p < 0.001) interaction effects and identified specific combinations of factors that result in superior mechanical performance. Sodium nitrite emerged as the most effective crosslinker, particularly when combined with extended incubation times. With the exception of the unincubated variant, the 30 min and 45 min heat-treated samples exhibited slightly higher internal bonding (IB) values compared to UF-bonded variants, while the 60 min treated was significantly higher. Conversely, heat treatment had a detrimental effect on the bisulfate-crosslinked variants, leading to a decrease in IB by -5%, -14%, and − 20% after 30 min, 45 min, and 60 min of heat treatment, respectively. The bisulfate and bisulfite-crosslinked variants exhibited lower IB values than the reference UF across different heat treatments. However, they were still competitive to the EN 319 requirement. Although the effect was less pronounced for bending strength (BS) and modulus of elasticity (MOE), a slight improvement was observed. Furthermore, with a BS ranging from 9.09 to 10.24 across the difference variants, the one-layer particleboards demonstrated a sufficiently high load bearing capacity. In summary, the study contributes to the development of plant protein-based adhesives as a viable option for wood composite manufacturing, highlighting the potential of diversifying protein sources for bio-adhesives in the wood industry and offering insights into the transition towards eco-friendly and sustainable materials. Moreover, it demonstrates that canola protein-based adhesives offer excellent mechanical properties and bonding performance, and are capable of competing with the synthetic adhesives in the production of interior-grade particleboards. However, further research is warranted to enhance the water resistance of the adhesive while preserving its excellent quality.
Data availability
The data used for the present publication is available upon request.
References
Adhikari BB, Appadu P, Chae M, Bressler DC (2017) Protein-based wood adhesives current trends of preparation and application. Bio-based Wood adhesives. CRC, pp 1–58
Alavi F, Chen L, Wang Z, Emam-Djomeh Z (2021) Consequences of heating under alkaline pH alone or in the presence of maltodextrin on solubility, emulsifying and foaming properties of faba bean protein. Food Hydrocoll 112:106335. https://doi.org/10.1016/j.foodhyd.2020.106335
Bandara N, Esparza Y, Wu J (2017) Exfoliating nanomaterials in canola protein derived adhesive improves strength and water resistance. RSC Adv 7:6743–6752. https://doi.org/10.1039/C6RA27470F
Barzegar M, Behrooz R, Mansouri HR, Najafi SK, Lorenz LF, Frihart CR (2020) Comparison of Canola and Soy Flour with added isocyanate as Wood adhesives. J Am Oil Chem Soc 97:1371–1383. https://doi.org/10.1002/aocs.12410
Barzegar M, Lorenz LF, Behrooz R, Frihart CR (2022) Improved Wood-Bond strengths using soy and Canola flours with pMDI and PAE. Polymers 14:1272. https://doi.org/10.3390/polym14071272
Bell J (1995) Meal and by-product utilization in animal nutrition. Brassica Oilseeds Prod Util. 301–337
Bérot S, Compoint JP, Larré C, Malabat C, Guéguen J (2005) Large scale purification of rapeseed proteins (Brassica napus L). J Chromatogr B 818:35–42. https://doi.org/10.1016/j.jchromb.2004.08.001
Bjorksten J (1951) Cross linkages in Protein Chemistry. Advances in Protein Chemistry. Elsevier, pp 343–381. https://doi.org/10.1016/S0065-3233(08)60507-0
Cárdenas-Oscanoa AJ, Tayo JLT, Huang, Caoxing, Huang, Chen, Euring M (2024) Discovering natural Fiber-insulation boards and natural adhesives, focused on a polylactic acid (PLA) application – a review. J Nat Fibers 21:2343371. https://doi.org/10.1080/15440478.2024.2343371
Chen C, Chen F, Liu B, Du Y, Liu C, Xin Y, Liu K (2019) Peanut meal-based wood adhesives enhanced by urea and epichlorohydrin. R Soc Open Sci 6:191154. https://doi.org/10.1098/rsos.191154
Chen C, Du Y, Chen F (2021) Effect of urea concentration on properties of peanut protein isolate, arachin and conarachin-based adhesives during urea-epichlorohydrin modification. R. Soc. Open Sci. 8, rsos.202227, 202227. https://doi.org/10.1098/rsos.202227
Chen X, Yang Z, Yang F, Zhang J, Pizzi A, Essawy H, Du G, Zhou X (2023) Development of easy-handled, formaldehyde-free, high-bonding performance bio-sourced wood adhesives by co-reaction of furfuryl alcohol and wheat gluten protein. Chem Eng J 462:142161. https://doi.org/10.1016/j.cej.2023.142161
Cheng E, Sun X (2006) Effects of wood-surface roughness, adhesive viscosity and processing pressure on adhesion strength of protein adhesive. J Adhes Sci Technol 20:997–1017. https://doi.org/10.1163/156856106777657779
Das A, Mukhopadhyay C (2009) Urea-mediated protein denaturation: a Consensus View. J Phys Chem B 113:12816–12824. https://doi.org/10.1021/jp906350s
Dunky M (2021) Wood adhesives based on Natural resources: a critical review: Part I. protein-based adhesives. In: Mittal KL (ed) Progress in adhesion and adhesives. Wiley, pp 203–336. https://doi.org/10.1002/9781119846703.ch8
Ericson ML, Rödin J, Lenman M, Glimelius K, Josefsson LG, Rask L (1986) Structure of the rapeseed 1.7 S storage protein, napin, and its precursor. J Biol Chem 261:14576–14581. https://doi.org/10.1016/S0021-9258(18)66909-1
Eslah F, Jonoobi M, Faezipour M, Afsharpour M, Enayati AA (2016) Preparation and development of a chemically modified bio-adhesive derived from soybean flour protein. Int J Adhes Adhes 71:48–54. https://doi.org/10.1016/j.ijadhadh.2016.08.011
Feldman D, Wood—chemistry, ultrastructure, reactions, by, Fengel D, Wegener G (1985) Walter de Gruyter, Berlin and New York, 1984, 613 pp. Price: 245 DM. J. Polym. Sci. Polym. Lett. Ed. 23, 601–602. https://doi.org/10.1002/pol.1985.130231112
Feng X, Li C, Jia X, Guo Y, Lei N, Hackman RM, Chen L, Zhou G (2016) Influence of sodium nitrite on protein oxidation and nitrosation of sausages subjected to processing and storage. Meat Sci 116:260–267. https://doi.org/10.1016/j.meatsci.2016.01.017
Frihart CR (2023) Protein adhesives – composition, structure and performance. In: Dunky M, Mittal KL (eds) Biobased adhesives. Wiley, pp 305–324. https://doi.org/10.1002/9781394175406.ch9
Frihart CR, Lorenz LF (2018) Protein adhesives. In: Pizzi A, Mittal K (eds) Handbook of Adhesive Technology. CRC Press, Taylor & Francis Group., Boca Ration, FL, pp 145–176
Frihart CR, Lorenz LF (2019) Specific oxidants improve the wood bonding strength of soy and other plant flours. J Polym Sci Part Polym Chem 57:1017–1023. https://doi.org/10.1002/pola.29357
Gehrig PM, Biemann K (1996) Assignment of the disulfide bonds in napin, a seed storage protein from Brassica napus, using matrix-assisted laser desorption ionization mass spectrometry. Pept Res 9:308–314
Hale K (2013) The Potential of Canola Protein for Bio-Based Wood Adhesive (Master´s Thesis). Kansas State University, Manhattan, KS, USA
He Z (ed) (2017) Bio-based Wood adhesives: Preparation, characterization, and testing, 1st edn. CRC, Boca Raton, FL. [2016] | A science publishers bookhttps://doi.org/10.1201/9781315369242
Hettiarachchy N.S., Kalapathy U (1998) Functional properties of Soy proteins. Funct Prop Proteins Lipids Whitaker J Al ACS Symp Ser Am Chem Soc Wash DC 1998 80–95. https://doi.org/10.1021/bk-1998-0708.ch006
Höglund A-S, Rödin J, Larsson E, Rask L (1992) Distribution of Napin and Cruciferin in developing rape seed embryos. Plant Physiol 98:509–515. https://doi.org/10.1104/pp.98.2.509
Hua L, Zhou R, Thirumalai D, Berne BJ (2008) Urea denaturation by stronger dispersion interactions with proteins than water implies a 2-stage unfolding. Proc Natl Acad Sci 105:16928–16933. https://doi.org/10.1073/pnas.0808427105
Huang J, Li K (2008) A New Soy Flour-based Adhesive for making Interior Type II plywood. J Am Oil Chem Soc 85:63–70. https://doi.org/10.1007/s11746-007-1162-1
Kallakas H, Plaza N, Crooks C, Turner D, Gargulak M, Arvanitis MA, Frihart CR, Hunt CG (2024) Effect of protein surface hydrophobicity and surface amines on Soy Adhesive Strength. Polymers 16:202. https://doi.org/10.3390/polym16020202
Kehr E, Schilling W (1965) Untersuchungen über die Eignung verschiedener Holzarten und -sortimente zur Herstellung von Spanplatten. 7. Mitteilung: Eiche, Aspe, Pappel, Hainbuche, Ulme, Lärche sowie als Vergleichsholzarten Fichte und Kiefer (Studies on the suitability of various Wood species and grades for Particleboard Production. Part 7: Oak, Aspen, Poplar, Hornbeam, Elm, Larch, and as comparative species Spruce and Pine) Holztechnologie 6:225–232
Klimov DK, Straub JE, Thirumalai D (2004) Aqueous urea solution destabilizes Aβ 16–22 oligomers. Proc Natl Acad Sci 101:14760–14765. https://doi.org/10.1073/pnas.0404570101
Li N, Qi G, Sun XS, Wang D (2012) Effects of Sodium Bisulfite on the Physicochemical and Adhesion Properties of Canola Protein Fractions. J Polym Environ 20:905–915. https://doi.org/10.1007/s10924-012-0490-x
Li N, Qi G, Sun XS, Wang D (2017) Canola protein and oil-based wood adhesives. Bio-Based Wood Adhes Prep Charact Test He Z Ed 111–139
Li C, Lei H, Wu Z, Xi X, Du G, Pizzi A (2022) Fully Biobased Adhesive from glucose and citric acid for plywood with high performance. ACS Appl Mater Interfaces 14:23859–23867. https://doi.org/10.1021/acsami.2c02859
Li C, Tang Y, Wang Y, Yuan X, Zhang B, Wu Z, Tian H (2022b) A Novel Environment-Friendly Adhesive based on recycling of Broussonetia papyrifera Leaf Forestry Waste protein. Forests 13:291. https://doi.org/10.3390/f13020291
Lifson S, Roig A (1961) On the theory of Helix—Coil transition in Polypeptides. J Chem Phys 34:1963–1974. https://doi.org/10.1063/1.1731802
Lorenz L, Frihart CR, Wescott JM (2007) Chromatographic analysis of the reaction of soy flour with Formaldehyde and Phenol for Wood adhesives. J Am Oil Chem Soc 84:769–776. https://doi.org/10.1007/s11746-007-1097-6
Lu ZX, He JF, Zhang YC, Bing DJ (2020) Composition, physicochemical properties of pea protein and its application in functional foods. Crit Rev Food Sci Nutr 60:2593–2605. https://doi.org/10.1080/10408398.2019.1651248
Lusas EW (2000) Oilseeds and oil-bearing materials. Handbook of Cereal Science and Technology, revised and expanded. CRC, pp 297–362
Manamperi WAR, Chang SKC, Ulven CA, Pryor SW (2010) Plastics from an Improved Canola protein isolate: Preparation and Properties. J Am Oil Chem Soc 87:909–915. https://doi.org/10.1007/s11746-010-1616-8
Manamperi WAR, Wiesenborn DP, Chang SKC, Pryor SW (2011) Effects of protein separation conditions on the Functional and Thermal properties of Canola protein isolates. J Food Sci 76. https://doi.org/10.1111/j.1750-3841.2011.02087.x
Mariod AA, Fadul H (2013) Gelatin, source, extraction and industrial applications. Acta Sci Pol Technol Aliment 12:135–147
Nardin M, Ward IM (1987) Influence of surface treatment on adhesion of polyethylene fibres. Mater Sci Technol 3:814–826. https://doi.org/10.1179/mst.1987.3.10.814
Nick Pace C, Scholtz M, J (1998) A Helix propensity scale based on experimental studies of peptides and proteins. Biophys J 75:422–427. https://doi.org/10.1016/S0006-3495(98)77529-0
Niemz P, Sonderegger W, Keplinger T, Jiang J, Lu J (2023) Physical properties of Wood and Wood-based materials. In: Niemz P, Teischinger A, Sandberg D (eds) Springer Handbook of Wood Science and Technology, Springer Handbooks. Springer International Publishing, Cham, pp 281–353. https://doi.org/10.1007/978-3-030-81315-4_6
Nur Hanani ZA, Roos YH, Kerry JP (2014) Use and application of gelatin as potential biodegradable packaging materials for food products. Int J Biol Macromol 71:94–102. https://doi.org/10.1016/j.ijbiomac.2014.04.027
Ostendorf K, Ahrens C, Beulshausen A, Tene Tayo JL, Euring M (2021) On the feasibility of a pMDI-Reduced production of Wood Fiber Insulation boards by means of Kraft Lignin and Ligneous Canola Hulls. Polymers 13:1088. https://doi.org/10.3390/polym13071088
Pace CN (1986) Determination and analysis of urea and guanidine hydrochloride denaturation curves. Methods in Enzymology. Elsevier, pp 266–280. https://doi.org/10.1016/0076-6879(86)31045-0
Pizzi A (2016) Synthetic adhesives for Wood fibers and composites: Chemistry and Technology. In: Belgacem N, Pizzi A (eds) Lignocellulosic fibers and Wood Handbook. Wiley, pp 245–276. https://doi.org/10.1002/9781118773727.ch9
Policardi F, Thebault M (2020) The buffer effect of different Wood species and the influence of Oak on Panel composites Binders. Polymers 12:1540. https://doi.org/10.3390/polym12071540
Sandermann W, Rothkamm M (1959) Über die Bestimmung Der pH-Werte Von Handelshölzern und deren Bedeutung für die Praxis (on the determination of pH values in Commercial Woods and their practical significance). Holz Als Roh- Werkst 17:433–440. https://doi.org/10.1007/BF02605386
Scheikl M, Dunky M (1998) Measurement of dynamic and staue contact angles on Wood for the determination of its Surface Tension and the penetration of liquids into the Wood Surface. Holzforschung 52:89–94. https://doi.org/10.1515/hfsg.1998.52.1.89
Schwenke KD, Raab B, Linow K-J, Pahtz W, Uhlig J (1981) Isolation of the 12 S globulin from rapeseed (Brassica napus L.) and characterization as a neutral protein on seed proteins. Part 13. Food Nahr 25:271–280. https://doi.org/10.1002/food.19810250307
Shi SQ, Xia C, Cai L (2017) Modification of soy-based adhesives to enhance the bonding performance. Bio-based Wood adhesives. CRC, pp 86–110
Singhal A, Karaca AC, Tyler R, Nickerson M (2016) Pulse Proteins: From Processing to Structure-Function Relationships, in: Goyal, A.K. (Ed.), Grain Legumes. InTech. https://doi.org/10.5772/64020
Solt P, Konnerth J, Gindl-Altmutter W, Kantner W, Moser J, Mitter R, Van Herwijnen HWG (2019) Technological performance of formaldehyde-free adhesive alternatives for particleboard industry. Int J Adhes Adhes 94:99–131. https://doi.org/10.1016/j.ijadhadh.2019.04.007
Stamm AJ (1964) Wood and cellulose science. Ronald Press Co. Michigan, USA
Sultana S, Ali ME, Ahamad MNU (2018) Gelatine, collagen, and single cell proteins as a natural and newly emerging food ingredients. Preparation and Processing of Religious and Cultural foods. Elsevier, pp 215–239. https://doi.org/10.1016/B978-0-08-101892-7.00011-0
Tene Tayo JL, Bettelhäuser RJ, Euring M (2022) Canola Meal as Raw Material for the development of Bio-adhesive for medium density fiberboards (MDFs) and Particleboards Production. Polymers 14:3554. https://doi.org/10.3390/polym14173554
Tzeng Y, Diosady LL, Rubin LJ (1990) Production of Canola protein materials by alkaline extraction, precipitation, and membrane Processing. J Food Sci 55:1147–1151. https://doi.org/10.1111/j.1365-2621.1990.tb01619.x
Vnučec D, Kutnar A, Goršek A (2017) Soy-based adhesives for wood-bonding – a review. J Adhes Sci Technol 31:910–931. https://doi.org/10.1080/01694243.2016.1237278
Wang C, Wu J, Bernard GM (2014) Preparation and characterization of canola protein isolate–poly(glycidyl methacrylate) conjugates: a bio-based adhesive. Ind Crops Prod 57:124–131. https://doi.org/10.1016/j.indcrop.2014.03.024
Wool RP, Sun XS (2005) Bio-based polymers and composites. Elsevier Academic, Amsterdam; Boston
Woolford G, Cassens RG, Greaser ML, Sebranek JG (1976) The fate of nitrite: Reaction with protein. J Food Sci 41:585–588. https://doi.org/10.1111/j.1365-2621.1976.tb00675.x
Wu J, Muir AD (2008) Comparative Structural, Emulsifying, and Biological properties of 2 Major Canola Proteins, Cruciferin and Napin. J Food Sci 73. https://doi.org/10.1111/j.1750-3841.2008.00675.x
Xiao G, Liang J, Wu Z, Lei H, Gong F, Gu W, Tu Y, Li D (2023) A Composite Whole-Biomass tannin–sucrose–soy protein Wood Adhesive with High Performance. Forests 14:1250. https://doi.org/10.3390/f14061250
Zeng H, Jin T, Shi S, Liu L, Guo H, Xie L, Chai X, Xu K, Du G, Zhang L (2023) Preparation a novel high performance glucose-based wood adhesive with hyperbranched cross-linked network by air oxidation. Polym Test 126:108157. https://doi.org/10.1016/j.polymertesting.2023.108157
Zhang Z, Hua Y (2007) Urea-modified soy globulin proteins (7S and 11S): Effect of Wettability and secondary structure on adhesion. J Am Oil Chem Soc 84:853–857. https://doi.org/10.1007/s11746-007-1108-7
Zhang Y, Wang X-M, Casilla R, Cooper P, Huang Z, Wang, Xiaodong (2010) Impact of curing condition on pH and alkalinity/acidity of structural wood adhesives. J Appl Polym Sci 117:2888–2898. https://doi.org/10.1002/app.32201
Acknowledgements
The authors’ gratitude goes to the company Pfleiderer GmbH (Neumarkt i.d.Opf., Deutschland) for kindly providing the wood chips used to produce the particleboards. We also acknowledge support by the Open Access Publication Funds of the Goettingen University.
Funding
This research was funded by AiF Projekt GmbH, funding number ZF4155613VS9 (Greenbond2Project, German–Swedish joint R &D project).
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conceptualization, L.T.T. and M.E.; Experiments, L.T.T., A.B., A: C-O and Z.C.; Formal data analysis and visualization, L.T.T. and A.C-O; Writing - original draft preparation, L.T.T.; Writing—review and editing, L.T.T., M.E., A: C-O and A.B.; Funding acquisition, M.E. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tene Tayo, L., Cárdenas-Oscanoa, A.J., Beulshausen, A. et al. Optimizing a canola-gelatine-urea bio-adhesive: effects of crosslinker and incubation time on the bonding performance. Eur. J. Wood Prod. (2024). https://doi.org/10.1007/s00107-024-02098-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00107-024-02098-8