Abstract
Purpose
In an effort to better manage critically ill patients hospitalised in the intensive care unit (ICU) after experiencing multiple traumas, the present study aimed to assess whether plasma levels of intestinal epithelial cell barrier proteins, including occludin, claudin-1, junctional adhesion molecule (JAM-1), tricellulin and zonulin, could be used as novel biomarkers. Additional potential markers such as intestinal fatty acid-binding protein (I-FABP), d-lactate, lipopolysaccharide (LPS) and citrulline were also evaluated. We also aimed to determine the possible relationships between the clinical, laboratory, and nutritional status of patients and the measured marker levels.
Methods
Plasma samples from 29 patients (first, second, fifth and tenth days in the ICU and on days 7, 30 and 60 after hospital discharge) and 23 controls were subjected to commercial enzyme-linked immunosorbent assay (ELISA) testing.
Results
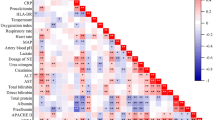
On first day (admission) and on the second day, plasma I-FABP, d-lactate, citrulline, occludin, claudin-1, tricellulin and zonulin levels were high in trauma patients and positively correlated with lactate, C-reactive protein (CRP), number of days of ICU hospitalisation, Acute Physiology and Chronic Health Evaluation II (APACHE II) score and daily Sequential Organ Failure Assessment (SOFA) scores (P < 0.05–P < 0.01).
Conclusion
The results of the present study showed that occludin, claudin-1, tricellulin and zonulin proteins, as well as I-FABP, d-lactate and citrulline, may be used as promising biomarkers for the evaluation of disease severity in critically ill trauma patients, despite the complexity of the analysis of various barrier markers. However, our results should be supported by future studies.



Similar content being viewed by others
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- APACHE II:
-
Acute Physiology and Chronic Health Evaluation II
- ASPEN:
-
American Society of Enteral and Parenteral Nutrition
- BMI:
-
Body mass index
- CRP:
-
C-reactive protein
- EDTA:
-
Ethylenediaminetetraacetic acid
- ELISA:
-
Enzyme-linked immunosorbent assay
- EN:
-
Enteral nutrition
- ESPEN:
-
European Society for Clinical Nutrition and Metabolism
- IBD:
-
Inflammatory bowel disease
- ICU:
-
Intensive care unit
- I-FABP:
-
Intestinal fatty acid-binding protein
- Ig:
-
Immunoglobulin
- IQR:
-
Interquartile range
- JAM:
-
Junctional adhesion molecule
- LPS:
-
Lipopolysaccharide
- mNUTRIC:
-
Modified nutrition risk in the critically ill
- PN:
-
Parenteral nutrition
- ROC:
-
Receiver operating characteristic
- SOFA:
-
Sequential Organ Failure Assessment
- TRISS:
-
Trauma and Injury Severity Score
References
Puleo F, Arvanitakis M, Van Gossum A, Preiser J-C. Gut failure in the ICU. Semin Respir Crit Care Med. 2011;32(5):626–38.
Ackland G, Grocott MP, Mythen MG. Understanding gastrointestinal perfusion in critical care: so near, and yet so far. Crit Care. 2000;4(5):1–13.
Denk S, Wiegner R, Hönes FM, Messerer DA, Radermacher P, Weiss M, Kalbitz M, Ehrnthaller C, Braumüller S, McCook O. Early detection of junctional adhesion molecule-1 (JAM-1) in the circulation after experimental and clinical polytrauma. Mediators Inflamm. 2015;2015:1–7.
Reintam Blaser A, Jakob SM, Starkopf J. Gastrointestinal failure in the ICU. Curr Opin Crit Care. 2016;22(2):128–41.
Li H, Chen Y, Huo F, Wang Y, Zhang D. Association between acute gastrointestinal injury and biomarkers of intestinal barrier function in critically ill patients. BMC Gastroenterol. 2017;17:1–8.
Asrani VM, Brown A, Huang W, Bissett I, Windsor JA. Gastrointestinal dysfunction in critical illness: a review of scoring tools. J Parenter Enter Nutr. 2020;44(2):182–96.
Reintam Blaser A, Preiser J-C, Fruhwald S, Wilmer A, Wernerman J, Benstoem C, Casaer MP, Starkopf J, van Zanten A, Rooyackers O. Gastrointestinal dysfunction in the critically ill: a systematic scoping review and research agenda proposed by the Section of Metabolism, Endocrinology and Nutrition of the European Society of Intensive Care Medicine. Crit Care. 2020;24:1–17.
Zhang X, Wang L, Chen D-C. Effect of rhubarb on gastrointestinal dysfunction in critically III patients: a retrospective study based on propensity score matching. Chin Med J. 2018;131(10):1142–50.
Blaser A, Padar M, Tang J, Dutton J, Forbes A. Citrulline and intestinal fatty acid-binding protein as biomarkers for gastrointestinal dysfunction in the critically ill. Anaesthesiol Intensive Ther. 2019;51(3):230–9.
Ewaschuk JB, Naylor JM, Zello GA. d-lactate in human and ruminant metabolism. J Nutr. 2005;135(7):1619–25.
Fragkos KC, Forbes A. Citrulline as a marker of intestinal function and absorption in clinical settings: a systematic review and meta-analysis. United Eur Gastroenterol J. 2018;6(2):181–91.
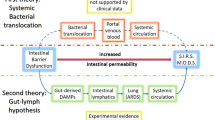
Ghosh SS, Wang J, Yannie PJ, Ghosh S. Intestinal barrier dysfunction, LPS translocation, and disease development. J Endocrine Soc. 2020;4(2):bvz039.
Monroe GR, van Eerde AM, Tessadori F, Duran KJ, Savelberg SM, van Alfen JC, Terhal PA, van der Crabben SN, Lichtenbelt KD, Fuchs SA. Identification of human D lactate dehydrogenase deficiency. Nat Commun. 2019;10(1):1477.
Moonen P-J, Blaser A, Starkopf J, Oudemans-van Straaten H, Van der Mullen J, Vermeulen G, Malbrain M. The black box revelation: monitoring gastrointestinal function. Anestezjol Intens Ter. 2019;50(1):73–82.
Peoc’h K, Nuzzo A, Guedj K, Paugam C, Corcos O. Diagnosis biomarkers in acute intestinal ischemic injury: so close, yet so far. Clin Chem Lab Med (CCLM). 2018;56(3):373–85.
Piton G, Manzon C, Cypriani B, Carbonnel F, Capellier G. Acute intestinal failure in critically ill patients: is plasma citrulline the right marker? Intensive Care Med. 2011;37:911–7.
Pohanka M. d-lactic acid as a metabolite: toxicology, diagnosis, and detection. BioMed Res Int. 2020;2020:1–9.
Shi H, Wu B, Wan J, Liu W, Su B. The role of serum intestinal fatty acid binding protein levels and d-lactate levels in the diagnosis of acute intestinal ischemia. Clin Res Hepatol Gastroenterol. 2015;39(3):373–8.
Voth M, Duchene M, Auner B, Lustenberger T, Relja B, Marzi I. I-FABP is a novel marker for the detection of intestinal injury in severely injured trauma patients. World J Surg. 2017;41:3120–7.
Garcia-Hernandez V, Quiros M, Nusrat A. Intestinal epithelial claudins: expression and regulation in homeostasis and inflammation. Ann N Y Acad Sci. 2017;1397(1):66–79.
Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124(1):3–20.
Krug SM, Fromm M. Special issue on “The tight junction and its proteins: more than just a barrier.” Int J Mol Sci. 2020;21:4612.
Lee B, Moon KM, Kim CY. Tight junction in the intestinal epithelium: its association with diseases and regulation by phytochemicals. J Immunol Res. 2018;2018:1–11.
Wang W, Uzzau S, Goldblum SE, Fasano A. Human zonulin, a potential modulator of intestinal tight junctions. J Cell Sci. 2000;113(24):4435–40.
Zeisel MB, Dhawan P, Baumert TF. Tight junction proteins in gastrointestinal and liver disease. Gut. 2019;68(3):547–61.
Halbgebauer R, Braun CK, Denk S, Mayer B, Cinelli P, Radermacher P, Wanner GA, Simmen H-P, Gebhard F, Rittirsch D. Hemorrhagic shock drives glycocalyx, barrier and organ dysfunction early after polytrauma. J Crit Care. 2018;44:229–37.
McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, McCarthy MS, Davanos E, Rice TW, Cresci GA. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). JPEN J Parenter Enteral Nutr. 2016;40(2):159–211.
Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, Hiesmayr M, Mayer K, Montejo JC, Pichard C. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48–79.
van Zanten ARH, De Waele E, Wischmeyer PE. Nutrition therapy and critical illness: practical guidance for the ICU, post-ICU, and long-term convalescence phases. Crit Care. 2019;23(1):1–10.
Osuka A, Kusuki H, Matsuura H, Shimizu K, Ogura H, Ueyama M. Acute intestinal damage following severe burn correlates with the development of multiple organ dysfunction syndrome: a prospective cohort study. Burns J Int Soc Burn Injuries. 2017;43(4):824–9.
Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol Rev. 2007;87(2):545–64.
Slifer ZM, Blikslager AT. The integral role of tight junction proteins in the repair of injured intestinal epithelium. Int J Mol Sci. 2020;21(3):972.
Kuo WT, Shen L, Zuo L, Shashikanth N, Ong M, Wu L, Zha J, Edelblum KL, Wang Y, Wang Y, Nilsen SP, Turner JR. Inflammation-induced occludin downregulation limits epithelial apoptosis by suppressing caspase-3 expression. Gastroenterology. 2019;157(5):1323–37.
Poritz LS, Harris LR, Kelly AA, Koltun WA. Increase in the tight junction protein claudin-1 in intestinal inflammation. Dig Dis Sci. 2011;56:2802–9.
Zhu L, Han J, Li L, Wang Y, Li Y, Zhang S. Claudin family participates in the pathogenesis of inflammatory bowel diseases and colitis-associated colorectal cancer. Front Immunol. 2019;10:1441.
Lameris AL, Huybers S, Kaukinen K, Mäkelä TH, Bindels RJ, Hoenderop JG, Nevalainen PI. Expression profiling of claudins in the human gastrointestinal tract in health and during inflammatory bowel disease. Scand J Gastroenterol. 2013;48(1):58–69.
Mineta K, Yamamoto Y, Yamazaki Y, Tanaka H, Tada Y, Saito K, Tamura A, Igarashi M, Endo T, Takeuchi K. Predicted expansion of the claudin multigene family. FEBS Lett. 2011;585(4):606–12.
Weber CR, Nalle SC, Tretiakova M, Rubin DT, Turner JR. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab Invest. 2008;88(10):1110–20.
Cording J, Günther R, Vigolo E, Tscheik C, Winkler L, Schlattner I, Lorenz D, Haseloff RF, Schmidt-Ott KM, Wolburg H. Redox regulation of cell contacts by tricellulin and occludin: redox-sensitive cysteine sites in tricellulin regulate both tri-and bicellular junctions in tissue barriers as shown in hypoxia and ischemia. Antioxid Redox Signal. 2015;23(13):1035–49.
Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171(6):939–45.
Krug S, Bojarski C, Fromm A, Lee I, Dames P, Richter J, Turner J, Fromm M, Schulzke J. Tricellulin is regulated via interleukin-13-receptor α2, affects macromolecule uptake, and is decreased in ulcerative colitis. Mucosal Immunol. 2018;11(2):345–56.
Fasano A. Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol. 2012;42:71–8.
Fasano A, Not T, Wang W, Uzzau S, Berti I, Tommasini A, Goldblum SE. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet. 2000;355(9214):1518–9.
Sturgeon C, Fasano A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers. 2016;4(4): e1251384.
Tripathi A, Lammers KM, Goldblum S, Shea-Donohue T, Netzel-Arnett S, Buzza MS, Antalis TM, Vogel SN, Zhao A, Yang S. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci. 2009;106(39):16799–804.
Klaus DA, Motal MC, Burger-Klepp U, Marschalek C, Schmidt EM, Lebherz-Eichinger D, Krenn CG, Roth GA. Increased plasma zonulin in patients with sepsis. Biochem Med. 2013;23(1):107–11.
Moreno-Navarrete JM, Sabater M, Ortega F, Ricart W, Fernandez-Real JM. Circulating zonulin, a marker of intestinal permeability, is increased in association with obesity-associated insulin resistance. PLoS ONE. 2012;7(5): e37160.
Żak-Gołąb A, Kocełak P, Aptekorz M, Zientara M, Juszczyk Ł, Martirosian G, Chudek J, Olszanecka-Glinianowicz M. Gut microbiota, microinflammation, metabolic profile, and zonulin concentration in obese and normal weight subjects. Int J Endocrinol. 2013;2013:1–9.
Robinson BD, Tharakan B, Lomas A, Wiggins-Dohlvik K, Alluri H, Shaji CA. Jupiter D, Isbell CL (2020) Exploring blood–brain barrier hyperpermeability and potential biomarkers in traumatic brain injury. In: Baylor University Medical Center proceedings: 2020. Taylor & Francis, pp 199–204
Blaser AR, Padar M, Mändul M, Elke G, Engel C, Fischer K, Giabicani M, Gold T, Hess B, Hiesmayr M, Jakob SM, Loudet CI, Meesters DM, Mongkolpun W, Paugam-Burtz C, Poeze M, Preiser JC, Renberg M, Rooijackers O, Tamme K, Wernerman J, Starkopf J. Development of the gastrointestinal dysfunction score (GIDS) for critically ill patients—a prospective multicenter observational study (iSOFA study). Clin Nutr. 2021;40(8):4932–40.
Funding
This work was funded by the Scientific Research Projects Coordination Unit of Erciyes University (Project ID: 8633).
Author information
Authors and Affiliations
Contributions
HD-A, SSE, PA-C, KB, HE, RU and KG: conception, design, and development of the study; acquisition, analysis and interpretation of the data; interpretation of results and writing and critical review of the manuscript. GGS, NTO, ST, TBA, AE, RCY and MS: acquisition, analysis, and interpretation of the data; writing and critical review of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no competing interests.
Ethics approval
Study design and protocol were approved by the University Medical Faculty Research Ethics Committee (No. 2018/525).
Consent for publication
Written informed consent for the use of blood samples was obtained from each patient (or a relative of the patient) and healthy volunteers before the collection of blood samples.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Donmez-Altuntas, H., Sahin Ergul, S., Altin-Celik, P. et al. Gut barrier protein levels in serial blood samples from critically ill trauma patients during and after intensive care unit stay. Eur J Trauma Emerg Surg 49, 2203–2213 (2023). https://doi.org/10.1007/s00068-023-02298-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00068-023-02298-6