Abstract
Purpose
The effect of systemic hemostatic agents initiated during pre-hospital care of severely injured patients with ongoing bleeding or traumatic brain injury (TBI) remains controversial. A systematic review and meta-analysis was therefore conducted to assess the effectiveness and safety of systemic hemostatic agents as an adjunctive therapy in people with major trauma and hemorrhage or TBI in the context of developing the Italian National Institute of Health guidelines on major trauma integrated management.
Methods
PubMed, Embase, and Cochrane Library databases were searched up to October 2021 for studies that investigated pre-hospital initiated treatment with systemic hemostatic agents. The certainty of evidence was evaluated with the Grading of Recommendations Assessment, Development, and Evaluation approach, and the quality of each study was determined with the Cochrane risk-of-bias tool. The primary outcome was overall mortality, and secondary outcomes included cause-specific mortality, health-related quality of life, any adverse effects and blood product use, hemorrhage expansion, and patient-reported outcomes.
Results
Five trials of tranexamic acid (TXA) met the inclusion criteria for this meta-analysis. With a high certainty of evidence, when compared to placebo TXA reduced mortality at 24 h (relative risk = 0.83, 95% confidence interval = 0.73–0.94) and at 1 month among trauma patients (0.91, 0.85–0.97). These results depend on the subgroup of patients with significant hemorrhage because in the subgroup of TBI there are no difference between TXA and placebo. TXA also reduced bleeding death and multiple organ failure whereas no difference in health-related quality of life.
Conclusion
Balancing benefits and harms, TXA initiated in the pre-hospital setting can be used for patients experiencing major trauma with significant hemorrhage since it reduces the risk of mortality at 24 h and one month with no difference in terms of adverse effects when compared to placebo. Considering the subgroup of severe TBI, no difference in mortality rate was found at 24 h and one month. These results highlight the need to conduct future studies to investigate the role of other systemic hemostatic agents in the pre-hospital settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe trauma is a major global public health issue [1]. According to the World Health Organization, the three most common causes of injury and violence-related deaths specifically road traffic accidents, suicides, and homicides. Furthermore, road traffic crashes and falls are the main causes of traumatic brain injury (TBI) [2]; intracranial bleeding is a possible complication of TBI [3], whose frequency depends on the injury severity [4]. Severely injured trauma patients are characterized by coagulation abnormalities and a substantially increased mortality rate [5]. Approximately 33% patients with TBI present with coagulopathy, which can increase the hemorrhage expansion and risk of death [4]. Hemorrhagic death generally occurs within the first few hours of injury (median time to hemorrhagic death is 2–2.6 h [6]), while late mortality is defined as death due to multiple organ failure or infection [7]. Over the last decade, increased awareness and improved pre-hospital care may have triggered a reduction in the number of severely injured patients with ongoing bleeding being transferred to a trauma center [8, 9]. Early detection and management of traumatic hemorrhage and TBI with intracranial hematoma can improve clinical outcomes [10], and, as suggested by the European guidelines, “current guidelines are needed for the implementation of evidence-based local protocols and algorithms together with parameters to assess key measures of bleeding control and outcome” [1, 9].
In trauma patients, interventions for bleeding may be required during the (1) acute phase (0–6 h from injury), with blood loss and shock; (2) intermediate phase (6–24 h from injury), with an increased risk of death due to severe TBI and concomitant physiologic impairment; and the (3) late phase, with the occurrence of inflammatory damages and/or complications [7]. Temporary measures to control hemorrhage in pre-hospital settings may be applied, such as the use of tourniquets, hemostatic dressings and pelvic binders, until definitive care is available. In non-compressible hemorrhage recent evidence suggests the resuscitative endovascular balloon occlusion of the aorta that however their use is still anedoctal [11]. Moreover, the use of pro-hemostatic agents has been suggested as an adjunctive measure to reduce bleeding and prevent trauma-induced coagulopathy [12] and to decrease the size of intracranial hematoma [2]. International guidelines strongly recommend the use of the synthetic lysine analog-tranexamic acid (TXA) in the early care of bleeding trauma patients at risk of significant hemorrhage [1, 5]. Thus, off-label early administration of this anti-fibrinolytic agent [1] has become a significant component of major hemorrhage protocols [12], but its infusion after 3 h from injury has been associated with the potential occurrence of adverse effects such as nausea, diarrhea, and stomach ache [1, 13]. Ideally, initial administration of TXA should be considered in the pre-hospital phase where possible, as evidenced by the decreasing survival benefit over time [14]. Current evidence suggests that early TXA hemostatic administration may improve outcomes in trauma patients both with hemorrhagic shock and intracranial bleeding [15]. Moreover, in cases of uncontrollable hemorrhage and coagulopathy, the off-label use of TXA has been suggested [1, 16]. While recombinant activated coagulation factor VII (rFVIIa) has been rarely investigated and used in the pre-hospital arena [17], TXA was tested by several published studies [18].
This systematic review aimed to assess the effectiveness and safety of systemic hemostatic agents as adjunctive treatment measures initiated in the pre-hospital setting and then continued in the in-hospital setting in patients with major trauma and hemorrhage or with TBI.
Methods
A systematic review was conducted to support the major trauma integrated management guideline panel of the Italian National Institute of Health (Istituto Superiore di Sanità, Sistema Nazionale Linee Guida) in formulating recommendations. Specifically, following the Grading of Recommendations Assessment, Development and Evaluation (GRADE)-ADOLOPMENT approach for guideline creation [19] adopted by the Istituto Superiore di Sanità methodological manual, the panel members decided to follow a structured and systematic adaptation and updating process of the recommended use of systemic hemostatic agents initiated in the pre-hospital setting from the National Institute for Health and Clinical Excellence (NICE) on major trauma (clinical guideline, NG39) [20]. The clinical question addressed in this systematic review was, “Is the use of systemic hemostatic agents clinically effective in improving outcomes in patients with confirmed or suspected hemorrhage in major trauma or with acute TBI?”.
Study design
The systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline (Supplemental Material 1) [21]. The study protocol is available at the following link: https://osf.io/cmdqk/.
Search strategy
Two professional librarians searched the Embase, PubMed (Medline), and the Cochrane Library databases for randomized controlled trials (RCTs) published up to October 12, 2021, that were related to the use of systemic hemostatic agents introduced in the pre-hospital environment among patients with confirmed or suspected hemorrhage or with acute TBI. Following the GRADE-ADOLOPMENT development process [19], the search strategy included in the high-quality NICE guideline on major trauma from 2015 [20] has been updated.
Full details of the search strategy are reported in Supplemental Material 2.
Study selection
Two independent authors (A.B. and G.P.) completed reference screening and study selection using the following pre-defined inclusion criteria: (1) population: children, young people, or adults who have experienced a suspected hemorrhage or TBI following a traumatic incident; (2) intervention: administration of a systemic hemostatic agent, (e.g., rFVIIa, TXA, fibrinogen concentrate, prothrombin complex concentrate, or another anti-fibrinolytic agent); (3) comparison: no treatment, placebo, standard care (e.g., crystalloids, blood components), or any other hemostatic agent; and (iv) setting: systemic hemostatic treatment initiated in the pre-hospital setting. Only RCTs were considered eligible for inclusion; case reports, editorials, and letters were excluded from the search.
When multiple publications for the same trial reported different outcomes or follow-up data, they were counted as a single publication. Discrepancies between reviewers were resolved by consulting a third author (O.C.).
Types of outcome measures and follow-up assessment
The primary outcome was the overall mortality (at 24 or 48 h or 1 month), while the secondary outcomes were cause-specific mortality at 1 month (e.g., multiple organ failure [MOF], head injury, hemorrhage), health-related quality of life; any adverse effect (e.g., MOF); blood product use; mortality at 12 months, hemorrhage expansion; and patient-reported outcomes.
Data extraction
Two independent authors (A.B. and G.P.) extracted data on publication year, country, and characteristics of the study population (e.g., age, Injury Severity Score [ISS], systolic blood pressure, heart rate, Glasgow Coma Scale, type of injury [blunt %], type of systemic hemostatic agents, and outcome data) using a standardized data-collection form in a spreadsheet format (Microsoft Excel, Redmond, WA, USA). Authors were contacted if the reported data were insufficient or unclear.
Statistical analysis
General characteristics were descriptively synthesized. When sufficient outcome data were available, cumulative analyses were performed. Treatment effects provided an estimate of the risk ratio (RR) for dichotomized outcomes and a mean difference (MD) or standardized mean difference (SMD) value when different outcome measurements were present for continuous outcomes, along with 95% confidence intervals (CI). Heterogeneity between study-specific estimates was tested using chi-squared statistics and measured with the I2 index and the Cochran’s Q test [22, 23]. A pooled estimate was obtained by fitting the DerSimonian and Laird random-effects model [24] when several studies were combined; conversely, a fixed-effects model was applied. Subgroup analyses were planned for (1) type of population (e.g., subjects with significant hemorrhage and subjects with TBI); (2) type of injury (e.g., blunt trauma, penetrating trauma); and (3) doses of systemic hemostatic agent when available. All tests were considered significant statistically for p < 0.05. For the primary outcome (i.e., mortality) the clinical relevance was assessed with the minimal important differences (MIDs) taken as risk ratios (RRs) of 0.75 and 1.25. For instance, the RR of 0.75 of mortality is taken as the line denoting the boundary between no clinically important effect and a clinically significant benefit, whilst the RR of 1.25 is taken as the line denoting the boundary between no clinically important effect and a clinically significant harm [20]. The analyses were performed using Review Manager Version 5.4 (Cochrane Collaboration, London, UK).
Risk-of-bias assessment (internal validity)
The Cochrane risk-of-bias tool was used to evaluate the internal validity of the included RCTs. The quality of the evidence was assessed using the GRADE methodology [25] (Supplemental Material 3).
Results
Study selection
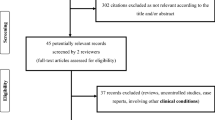
Overall, 1982 records were identified by updating the search. Ten references met the study eligibility criteria, including eight primary studies (i.e., RCTs) [2, 26,27,28,29,30,31] and two systematic reviews [32, 33], out of which three studies were extracted [4, 34]. One study [5] was already included in the NICE guideline. In addition, six references were sourced from ClinicalTrials.gov [35,36,37,38,39,40]. After performing an overlapping evaluation between studies included in the retrieved systematic reviews and primary studies from the updated search strategy, ongoing trials, and the one [5] already included in the NICE guideline, 17 studies related to seven RCTs were included (Fig. 1). In Supplemental Material 2, all included publications related to the five RCTs are listed, namely CRASH-2 [4, 5, 27, 29, 34,35,36,37,38,39,40], CRASH-3 [2, 26], the TXA trial [28, 41], STAAMP [30], and TAMPITI [31]. No eligible study was found on the use of fibrinogen concentrates, recombinant activated coagulation factor VII, prothrombin complex concentrates, or other anti-fibrinolytic agents in the pre-hospital setting.
General characteristics
Overall, five RCTs assessed the comparison of TXA and placebo (Supplemental Material 2). The median ISS across studies ranged from a minimum of 12 (interquartile range [IQR] = 5–22) to a maximum of 38.7 (IQR = 25–52.4). Blunt trauma was the most representative feature of patients across studies. In particular, TXA versus placebo was investigated in patients with significant hemorrhage [5, 31, 36, 40] or who were at risk of hemorrhage [30], in patients with isolated TBI [2, 26, 41], and in those with brain injury and significant hemorrhage [4, 34]. The general and clinical characteristics of patients are presented in Table 1.
Overall mortality
All included RCTs (n = 5) reported the overall mortality data. The CRASH-2 and STAAMP trials showed mortality at 24 h, while five RCTs (CRASH-2, CRASH-3, STAAMP, TAMPITI, and the TXA trial) evaluated overall mortality at 1 month.
TXA versus placebo
A statistically significant difference including clinical relevance between TXA and placebo for overall mortality at 24 h was observed (RR = 0.83, 95% CI = 0.74–0.95; two studies, 21,030 patients; Fig. 2A), whereas a statistically but not clinically significant reduction was found at 1 month in the TXA group (RR = 0.93, 95% CI = 0.88–0.97; five studies, 34,873 patients, Fig. 2B). The absolute effects with TXA shown 8 and 12 fewer deaths per 1.000 at 24 h and 1 month, respectively.
In the subgroup analysis for the type of population (Fig. S1 in Supplemental Material 4), TXA did not reduce the overall mortality at 1 month in patients with TBI (RR = 0.96, 95% CI = 0.89–1.03; two studies, 13,703 participants) or those with TBI and significant hemorrhage (RR = 0.72; 95% CI = 0.49–1.05, three studies, 587 participants) compared to placebo; however, a reduction was registered among trauma patients with significant hemorrhage (RR = 0.91, 95% CI = 0.85–0.97; two studies, 20,853 participants; Table S2 in Supplemental Material 4).
Secondary outcome
Descriptive tables and quantitative analyses for the following outcomes are shown in Supplemental Material 4.
-
Cause-specific mortality: Bleeding death at 1 month was significantly reduced in patients who received TXA compared to placebo, whereas no differences between the groups at 1 month were detected for MOF, head injury, pulmonary embolism, and sepsis (Table S3 and Figs. S3–S5 in Supplemental Material 4).
-
Health-related quality of life: There was no clear effect of TXA on disability among survivors, as evaluated by the Disability Rating Scale [42] in two RCTs [2, 28] (Fig. S6 in Supplemental Material 4).
-
Adverse effects: Reported data on sepsis, pulmonary embolism, myocardial infarction or stroke, renal failure, seizures, gastrointestinal bleeding, acute respiratory distress syndrome (ARDS), MOF, and vascular occlusive events did not demonstrate an increased risk with the use of TXA compared to placebo (Table S4 and Figs. S7–S16 in Supplemental Material 4).
-
Blood product use: There was no difference in blood, platelet, or plasma transfusions between groups (Table S5 in Supplemental Material 4).
-
Other secondary outcomes: None of the RCTs reported the mortality at 12 months or patient-reported outcomes, and no significant reduction was detected regarding hemorrhage expansion (Table S6 in Supplemental Material 4).
The above results suggest a higher safety profile of TXA therapy without a significant difference in complications between the two groups.
Internal validity and certainty of evidence
Five RCTs (CRASH-2, CRASH-3, STAAMP, TAMPITI, and TXA trial) were judged to have good methodological quality (Supplemental Material 3). The certainty of evidence across the RCTs ranged from very low to high quality (Table 2).
Discussion
To our knowledge, this is the first comprehensive systematic review with a meta-analysis assessing the certainty of evidence by the GRADE approach to prove the efficacy of systemic hemostatic agents as an adjunctive treatment initiated in the pre-hospital setting among traumatic patients with (1) hemorrhage, (2) TBI, or (3) hemorrhage and TBI. With a high certainty of evidence, TXA is associated with a lower risk of mortality of 17% at 24 h (RR = 0.83, 95% CI = 0.74–0.95) and 7% at one month (RR = 0.93, 95% CI 0.88–0.97) when compared to placebo. Although the clinical relevance could be questionable, the anticipated absolute effects at 24 h and 1 month anyway shown a reduction of mortality of 8 and 12 averted deaths per 1000 which may be relevant in a life threatening conditions (i.e., major trauma). Considering the subgroup of severe TBI at 24 h and one month, both failed to find any difference between groups.
Difference between patients with severe hemorrhage and TBI may be explained by the timing of anti-fibrinolytic administration and the severity of TBI [2, 26]. TBI, commonly characterized by intracranial hemorrhage, is particularly concerning in this context because it can progress or gradually worsen after hospitalization [43]. One-third of patients affected by severe TBI may develop coagulopathy because of the release of brain phospholipids and tissue factors [44]. However, a recent meta-analysis did not show a strong prevalence of coagulopathy in TBI patients compared to those with injuries in other areas of the body or multiple injuries with TBI [45]. Especially in the early stages [46], TXA administration tends to reduce hemorrhage expansion and mortality caused by bleeding in TBI patients [34]; nevertheless, those affected by intracranial bleeding and neuropathological abnormalities did not show significant benefits of anti-fibrinolytic treatment [26]. Within 24 h of injury deaths are more likely to occur because of excessive bleeding [2].
We can use 24-h mortality as a proxy of the overall mortality effect [6], since the ideal endpoint in literature is considered as mortality within the first 6 h after injury [14, 47], in fact the CRASH-2, which represents the most contribution (96.5% of weight of meta-analysis), administered the TXA within 3 h from injury. The effect of systemic hemostatic agents depends on the time between injury and the onset of treatment, which suggests there is a protective effect of these medications on early deaths related to hemorrhage [48].
The treatment benefit is time-dependent, the benefits of TXA therapy seen in the first 3 h may be explained by the increasing levels of plasminogen activator inhibitor-1 and a reduction in fibrinolysis. In CRASH-2, when TXA was administered within 3 h of injury, the risk of death due to bleeding was reduced [29]. However, in the late phase of TXA administration, adverse effects, especially thrombotic disseminated intravascular coagulation, was likely to increase, along with uncontrolled bleeding [29] and a higher risk of mortality [49].
Therefore, its administration may be contraindicated [36]. However, we found no differences between TXA and placebo in terms of adverse effects. In addition, many guidelines have focused on TXA administration in severely injured or bleeding patients [20]. The British Committee for Standards in Hematology recommends the administration of TXA in adult trauma patients with, or who are at risk of, bleeding as early as possible after injury [50], and the STOP the Bleeding Campaign in Europe also recommends its use in bleeding trauma patients within 3 h after an injury and those “en route” to the hospital [51]. On the basis of existing evidence, TXA was also included on the World Health Organization’s list of essential medicine for the reduction of death among adult patients with trauma and a significant risk of ongoing hemorrhage [52].
Our results are in agreement with those in the current scientific literature. Specifically, meta-analyses and systematic reviews conducted in the pre-hospital or the hospital setting found that TXA reduces the risk of death from all causes by about 20% [44, 53, 54] and less hemorrhagic expansion [44] among TBI patients [32, 46]; yet some results were discrepant with respect to functional status [32, 44, 54, 55]. No evidence was detected for other adverse events [32, 44, 46, 55], although high-quality RCTs reported reduced vascular occlusive events [44]. However, confounders may have influenced the results, such as blood transfusions realized prior to TXA treatment, although this mechanism is still unknown [53].
This study has some limitations. First, survival bias may be present when patients die before the administration of treatment; thus, the results might not be generalizable across the trauma population [56]. Second, cause-specific mortality can be affected by a subjective and misclassified evaluation (e.g., an in-hospital death for hemorrhage may be caused by hemorrhage and severe TBI [6]). Third, clinical conditions (e.g., cardiac arrest) may have strongly influenced the treatment indication. Statistical analyses may not have taken into account the time-varying treatment effect by assuming uniform effects over time, nor adequately considered risk factors [56], or the study outcomes (e.g., reduction of mortality) [39]. Thus, appropriate strategies may reduce these type of biases [56]. Some studies also did not specify the type of trauma (blunt or penetrating) and did not categorize patients based on their trauma severity, while others performed secondary analyses or re-analyses (e.g., restriction to TBI patients). These factors may have limited the subgroup analysis. Fourth, inclusion criteria were narrow, aiming to balance pros and cons. We considered eligible all RCTs that investigated several interventions delivered in any setting (civilian and military). Thus, although we were able to offer the highest level of evidence (RCTs with high certainty of evidence) achieving more consistency in the interpretation of findings, this approach limited the results as we did not find studies in military setting or studies involving fibrinogen concentrates, prothrombin complex concentrate, or other anti-fibrinolytic agents. The inclusion observational evidence (e.g., non-randomized interventional studies) a different pragmatic approach have been found. Early patient randomization may not have prevented the performance of stratified analysis on the anatomical location of bleeding or other injury [36]. Moreover, difficult diagnoses of traumatic hemorrhages might have reduced the statistical power analysis focused on the anti-fibrinolytic effect on bleeding mortality or the need for blood transfusions [5]. Fifth, heterogeneity was found in reporting the average time from injury to anti-fibrinolytic administration, as well as the time of data collection regarding outcome. Last, the complexity of the trauma cohorts and different choices made by trauma teams in their management across various countries may have affected the overall analyses [30].
Conclusion
Balancing benefits and harms, tranexamic acid initiated in the pre-hospital setting can be used for patients experiencing major trauma with significant hemorrhage. Considering the ideal endpoint in literature is the administration of TXA within 6 h from injury, TXA statistically and clinically reduces mortality rate in trauma patients at 24 h. This result is still statistically significant at 1 month. These results are mainly dragged by the subgroup of patients with significant hemorrhage because in TBI patients subgroup there are no difference between TXA and control. Little-to-no difference in terms of adverse effects was found when comparing TXA and placebo. Further research is needed to investigate the role of other systemic hemostatic agents in the pre-hospital settings.
Supplemental Material 1. PRISMA 2009 Checklist.
Supplemental Material 2. Search strategy and study references.
Supplemental Material 3. Internal Validity and Certainty of the evidence.
Supplemental Material 4. Additional results: primary and secondary outcomes.
References
Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Crit Care. 2016;20:100.
CRASH-3 Trial Collaborators. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. Lancet. 2019;394:1713–23.
Perel P, Roberts I, Bouamra O, Woodford M, Mooney J, Lecky F. Intracranial bleeding in patients with traumatic brain injury: a prognostic study. BMC Emerg Med. 2009;9:15.
CRASH-2 Collaborators, Intracranial Bleeding Study. Effect of tranexamic acid in traumatic brain injury: a nested randomised, placebo controlled trial (CRASH-2 Intracranial Bleeding Study). BMJ. 2011;343:d3795.
CRASH-2 Trial Collaborators, Shakur H, Roberts I, Bautista R, Caballero J, Coats T, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376:23–32.
Fox EE, Holcomb JB, Wade CE, Bulger EM, Tilley BC, PROPPR Study Group. Earlier endpoints are required for hemorrhagic shock trials among severely injured patients. Shock. 2017;47:567–73.
Holcomb JB, Moore EE, Sperry JL, Jansen JO, Schreiber MA, Del Junco DJ, et al. Evidence-based and clinically relevant outcomes for hemorrhage control trauma trials. Ann Surg. 2021;273:395–401.
Driessen A, Fröhlich M, Schäfer N, Mutschler M, Defosse JM, Brockamp T, et al. Prehospital volume resuscitation—did evidence defeat the crystalloid dogma? An analysis of the TraumaRegister DGU® 2002–2012. Scand J Trauma Resusc Emerg Med. 2016;24:42.
Maegele M, Nardi G, Schöchl H. Hemotherapy algorithm for the management of trauma-induced coagulopathy: the German and European perspective. Curr Opin Anaesthesiol. 2017;30:257–64.
Huber-Wagner S, Lefering R, Qvick M, Kay MV, Paffrath T, Mutschler W, et al. Outcome in 757 severely injured patients with traumatic cardiorespiratory arrest. Resuscitation. 2007;75:276–85.
Bulger EM, Perina DG, Qasim Z, Beldowicz B, Brenner M, Guyette F, et al. Clinical use of resuscitative endovascular balloon occlusion of the aorta (REBOA) in civilian trauma systems in the USA, 2019: a joint statement from the American College of Surgeons Committee on Trauma, the American College of Emergency Physicians, the National Association of Emergency Medical Services Physicians and the National Association of Emergency Medical Technicians. Trauma Surg Acute Care Open. 2019;4: e000376.
Vulliamy P, Thaventhiran AJ, Davenport RA. What’s new for trauma haemorrhage management? Br J Hosp Med (Lond). 2019;80:268–73.
Chauncey JM, Wieters JS. Tranexamic Acid [Internet]. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022 [citato 2022 set 1]. http://www.ncbi.nlm.nih.gov/books/NBK532909/
Gayet-Ageron A, Prieto-Merino D, Ker K, Shakur H, Ageron FX, Roberts I, et al. Effect of treatment delay on the effectiveness and safety of antifibrinolytics in acute severe haemorrhage: a meta-analysis of individual patient-level data from 40 138 bleeding patients. Lancet. 2018;391:125–32.
Liu Z, Ayyagari RC, Martinez Monegro EY, Stansbury LG, Arbabi S, Bulger EM, et al. Blood component use and injury characteristics of acute trauma patients arriving from the scene of injury or as transfers to a large, mature US Level 1 trauma center serving a large, geographically diverse region. Transfusion. 2021;61:3139–49.
Johnson SM, Tsang D, Dansby M, Allen C. New and off-label uses of tranexamic acid. AACN Adv Crit Care. 2021;32:237–42.
Kim B, Haque A, Arnaud FG, Teranishi K, Steinbach T, Auker CR, et al. Use of recombinant factor VIIa (rFVIIa) as pre-hospital treatment in a swine model of fluid percussion traumatic brain injury. J Emerg Trauma Shock. 2014;7:102–11.
Napolitano LM. Prehospital tranexamic acid: what is the current evidence? Trauma Surg Acute Care Open. 2017;2: e000056.
Schünemann HJ, Wiercioch W, Brozek J, Etxeandia-Ikobaltzeta I, Mustafa RA, Manja V, et al. GRADE evidence to decision (EtD) frameworks for adoption, adaptation, and de novo development of trustworthy recommendations: GRADE-ADOLOPMENT. J Clin Epidemiol. 2017;81:101–10.
National Clinical Guideline Centre (UK). Major Trauma: Assessment and Initial Management. London: National Institute for Health and Care Excellence (UK); 2016. (NICE Guideline, No. 39.) 10, assessment and management of haemorrhage. https://www.ncbi.nlm.nih.gov/books/NBK368100/.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097.
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–29.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–6.
CRASH-3 Intracranial Bleeding Mechanistic Study Collaborators. Tranexamic acid in traumatic brain injury: an explanatory study nested within the CRASH-3 trial. Eur J Trauma Emerg Surg. 2021;47:261–8.
Nishijima DK, Kuppermann N, Roberts I, VanBuren JM, Tancredi DJ. The effect of tranexamic acid on functional outcomes: an exploratory analysis of the CRASH-2 randomized controlled trial. Ann Emerg Med. 2019;74:79–87.
Prehospital Tranexamic Acid Use for Traumatic Brain Injury (TXA). https://clinicaltrials.gov/ct2/show/NCT01990768. Accessed Oct 2020.
Roberts I, Edwards P, Prieto D, Joshi M, Mahmood A, Ker K, et al. Tranexamic acid in bleeding trauma patients: an exploration of benefits and harms. Trials. 2017;18:48.
Guyette FX, Brown JB, Zenati MS, Early-Young BJ, Adams PW, Eastridge BJ, et al. Tranexamic acid during prehospital transport in patients at risk for hemorrhage after injury: a double-blind, placebo-controlled, randomized clinical trial. JAMA Surg. 2020.
Spinella PC, Thomas KA, Turnbull IR, Fuchs A, Bochicchio K, Schuerer D, et al. The immunologic effect of early intravenous two and four gram bolus dosing of tranexamic acid compared to placebo in patients with severe traumatic bleeding (TAMPITI): a randomized, double-blind, placebo-controlled, single-center trial. Front Immunol. 2020;11:2085.
Chen H, Chen M. The efficacy of tranexamic acid for brain injury: a meta-analysis of randomized controlled trials. Am J Emerg Med. 2020;38:364–70.
Perel P, Roberts I, Shakur H, Thinkhamrop B, Phuenpathom N, Yutthakasemsunt S. Haemostatic drugs for traumatic brain injury. Cochrane Database Syst Rev. 2010;CD007877.
Perel P, Al-Shahi Salman R, Kawahara T, Morris Z, Prieto-Merino D, Roberts I, et al. CRASH-2 (Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage) intracranial bleeding study: the effect of tranexamic acid in traumatic brain injury—a nested randomised, placebo-controlled trial. Health Technol Assess. 2012;16:iii–xii, 1–54.
Brief details of the trial protocol (da http://www.crash2.lshtm.ac.uk/): a large randomised placebo controlled trial among trauma patients with or at risk of significant haemorrhage, of the effects of antifibrinolytic treatment on death and transfusion requirement.
CRASH-2 Collaborators, Roberts I, Shakur H, Afolabi A, Brohi K, Coats T, et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet. 2011;377:1096–101 (1101.e1–2).
Guerriero C, Cairns J, Perel P, Shakur H, Roberts I, CRASH 2 trial collaborators. Cost-effectiveness analysis of administering tranexamic acid to bleeding trauma patients using evidence from the CRASH-2 trial. PLoS One. 2011;6:e18987.
Meurer WJ. Tranexamic acid reduced mortality in trauma patients who were bleeding or at risk for bleeding. Ann Intern Med. 2013;159:JC3.
Roberts I, Prieto-Merino D, Manno D. Mechanism of action of tranexamic acid in bleeding trauma patients: an exploratory analysis of data from the CRASH-2 trial. Crit Care. 2014;18:685.
Roberts I, Shakur H, Coats T, Hunt B, Balogun E, Barnetson L, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess. 2013;17:1–79.
Rowell SE, Meier EN, McKnight B, Kannas D, May S, Sheehan K, et al. Effect of out-of-hospital tranexamic acid vs placebo on 6-month functional neurologic outcomes in patients with moderate or severe traumatic brain injury. JAMA. 2020;324:961–74.
Rappaport M, Hall KM, Hopkins K, Belleza T, Cope DN. Disability rating scale for severe head trauma: coma to community. Arch Phys Med Rehabil. 1982;63:118–23.
Ker K, Roberts I, Shakur H, Coats TJ. Antifibrinolytic drugs for acute traumatic injury. Cochrane Database Syst Rev. 2015;CD004896.
July J, Pranata R. Tranexamic acid is associated with reduced mortality, hemorrhagic expansion, and vascular occlusive events in traumatic brain injury—meta-analysis of randomized controlled trials. BMC Neurol. 2020;20(1):1–11.
Yuan Q, Sun YR, Wu X, Yu J, Li ZQ, Du ZY, et al. Coagulopathy in traumatic brain injury and its correlation with progressive hemorrhagic injury: a systematic review and meta-analysis. J Neurotrauma. 2016;33:1279–91.
Weng S, Wang W, Wei Q, Lan H, Su J, Xu Y. Effect of tranexamic acid in patients with traumatic brain injury: a systematic review and meta-analysis. World Neurosurg. 2019;123:128–35.
Spinella PC, El Kassar N, Cap AP, Kindzelski AL, Almond CS, Barkun A, et al. Recommended primary outcomes for clinical trials evaluating hemostatic blood products and agents in patients with bleeding. Proceedings of a National Heart Lung and Blood Institute and US Department of Defense Consensus Conference. J Trauma Acute Care Surg. 2021;91:S19–25.
Eastridge BJ, Holcomb JB, Shackelford S. Outcomes of traumatic hemorrhagic shock and the epidemiology of preventable death from injury. Transfusion. 2019;59:1423–8.
Nishida T, Kinoshita T, Yamakawa K. Tranexamic acid and trauma-induced coagulopathy. J Intensive Care. 2017;5:5.
Hunt BJ, Allard S, Keeling D, Norfolk D, Stanworth SJ, Pendry K, et al. A practical guideline for the haematological management of major haemorrhage. Br J Haematol. 2015;170:788–803.
Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, et al. The STOP the bleeding campaign. Crit Care. 2013;17:136.
World Health Organization. The selection and use of essential medicines. World Health Organ Tech Rep Ser 2014;i–xiv, 1–219, back cover.
Huebner BR, Dorlac WC, Cribari C. Tranexamic acid use in prehospital uncontrolled hemorrhage. Wilderness Environ Med. 2017;28:S50-60.
Morales-Cané I, López-Soto PJ, Rodríguez-Borrego MA. Tranexamic acid in trauma patients in the emergency department: systematic review and meta-analysis. Emergencias. 2019;31:261–9.
Zehtabchi S, Abdel Baki SG, Falzon L, Nishijima DK. Tranexamic acid for traumatic brain injury: a systematic review and meta-analysis. Am J Emerg Med. 2014;32:1503–9.
del Junco DJ, Fox EE, Camp EA, Rahbar MH, Holcomb JB, PROMMTT Study Group. Seven deadly sins in trauma outcomes research: an epidemiologic post mortem for major causes of bias. J Trauma Acute Care Surg. 2013;75:S97–103.
Acknowledgements
The Italian National Institute of Health Guideline Working Group on Major Trauma includes Carlo Coniglio (car.coniglio@gmail.com), Elvio De Blasio (elvio.deblasio@gmail.com), Gaddo Flego (gaddo.flego@oeige.org), Massimo Geraci (mageraci@hotmail.it), Giulio Maccauro (giulio.maccauro@policlinicogemelli.it), Antonio Rampoldi (antoniogaetano.rampoldi@ospedaleniguarda.it), Federico Santolini (federico.santolini@hsanmartino.it), Claudio Tacconi (claudiotacconi80@gmail.com), Gregorio Tugnoli (gregorio.tugnoli@ausl.bologna.it), Nino Stocchetti (nino.stocchetti@unimi.it), Andrea Fabbri (dr.andrea.fabbri@gmail.com), Maria Pia Ruggeri (mpruggieri@hsangiovanni.roma.it). We thank Maurella Della Seta, Scilla Pizzarelli, Rosaria Rosanna Cammarano and the Istituto Superiore di Sanità documentalists for performing the search strategy and, Alessia Medici and Alessandro Mazzola for the administrative and organizational support. We thank the Charlesworth Author Services for the English Academic Editing.
Author information
Authors and Affiliations
Consortia
Contributions
OC and PI, conceived the idea; AB, GP, GC, and SG developed the study design and performed the data collection, analysis, acquisition of data, interpretation of data; AB, GP, GC, SG, and OC drafted the article; AN, DC, DD, AJF, LI, RL, KS, and SG interpreted the data and revised the paper critically for important intellectual content; OC and PI take responsibility for the paper as a whole. All authors approved the final version to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interests exist.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Membership of the Italian National Institute of Health guideline working group is listed in the Acknowledgments.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Biffi, A., Porcu, G., Castellini, G. et al. Systemic hemostatic agents initiated in trauma patients in the pre-hospital setting: a systematic review. Eur J Trauma Emerg Surg 49, 1259–1270 (2023). https://doi.org/10.1007/s00068-022-02185-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00068-022-02185-6