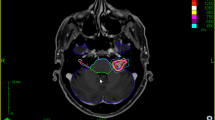
Abstract
Purpose
Data concerning the clinical usefulness of steady-state sequences (SSS) for vestibular schwannomas (VS) after linear accelerator (LINAC) stereotactic radiosurgery (SRS) or stereotactic radiotherapy (SRT) are scarce. The aim of the study was to investigate whether SSS provide an additional useful follow-up (FU) tool to the established thin-layered T1 sequences with contrast enhancement.
Methods
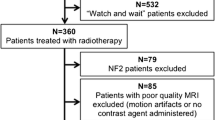
Pre- and post-treatment SSS were identified in 45 consecutive VS patients (2012–2016) with a standardized FU protocol including SSS at 2–3 months and 6 months/yearly in our prospective database and were retrospectively re-evaluated. The SSS were used throughout for the segmentation of the cochlea and partly of the trigeminal nerve in the treatment planning. Data analysis included signal conversion in SSS and possible correlation with neuro-otological outcome and volumetric assessment after a certain time interval.
Results
The series included 42 SRS and 3 SRT patients (31 female/14 male; mean age 59.3 years, range: 25–81 years). An SSS signal conversion was observed in 20 tumors (44.4%) within a mean time of 11 months (range: 7–15 months). Mean FU time was 26 months (median of 4 FU visits) and demonstrated tumor volume shrinkage in 29 cases (64.4%) correlating with FU time (p = 0.07). The incidence rate of combined shrinkage and signal conversion (48.3%) compared to those without signal conversion (51.7%) did not differ significantly (p = 0.49). In case of an early signal conversion at the first FU, a weak statistical significance (p = 0.05) for a higher shrinkage rate of VS with signal conversion was found. Side effects in cases with signal conversion (9/20, 45%) were more frequently than without signal conversion (6/25, 24%) without reaching statistical significance (p = 0.13).
Conclusion
Our data confirmed the usefulness of SSS for anatomical segmentation of VS in LINAC-SRS/SRT treatment planning and add data supporting their potential as an adjunctive FU option in VS patients.
Zusammenfassung
Zielsetzung
Daten, die den klinischen Nutzen von Steady-State-Sequenzen (SSS) für Vestibularisschwannome (VS) nach einer LINAC („linear accelerator“) stereotaktischen Radiochirurgie (SRS) oder Radiotherapie (SRT) als Verlaufsparameter zeigen, sind selten. Ziel der Studie war es, zu untersuchen, ob SSS auch bei Follow-up(FU)-Untersuchungen neben den etablierten dünnschichtigen T1-Sequenzen mit Kontrastmittel nützlich sind.
Methoden
Bei 45 VS-Patienten (2012–2016) wurden SSS vor und nach Behandlung durchgeführt; ein standardisiertes FU-Schema sah SSS nach 2–3 Monaten, einem halben Jahr und jährlich vor. Die SSS wurden durchgehend für die Segmentierung der Cochlea und zum Teil auch des N. trigeminus bei der Bestrahlungsplanung eingesetzt. Die Ergebnisse wurden in einer prospektiven Datenbank erfasst und retrospektiv ausgewertet. Die Datenanalyse beinhaltete Signalkonversionen in den SSS und ihre möglichen Korrelationen mit neurootologischen Befunden und volumetrischen Veränderungen nach bestimmten Zeitintervallen.
Ergebnisse
Die Serie umfasste 42 SRS- und 3 SRT-Patienten (31 Frauen/14 Männer; mittleres Alter 59,3 Jahre; Spanne 25–81 Jahre). Eine SSS-Signalkonversion wurde in 20 Tumoren (44,4%) nach einem mittleren Zeitraum von 11 Monaten (Spanne 7–15 Monate) beobachtet. Die mittlere FU-Zeit betrug 26 Monate (mit einem Median von 4 FU-Terminen) und zeigte eine Tumorschrumpfung in 29 Fällen (64,4%), die mit der FU schwach korrelierte (p = 0,07). Die Häufigkeit des Auftretens von kombinierten Schrumpfungen und Signalkonversionen (48,3%) verglichen mit denen ohne Signalkonversion (51,7%) unterschied sich nicht signifikant (p = 0,49). Im Falle einer frühen Signalkonversion bereits bei der ersten FU konnte jedoch eine schwache statistische Signifikanz (p = 0,05) für eine höhere VS-Schrumpfungsrate festgestellt werden. Nebenwirkungen in Fällen mit Signalkonversion (9/20, 45%) waren etwas häufiger als bei Patienten ohne Signalkonversion (6/25, 24%), jedoch ohne statistische Signifikanz (p = 0,13).
Schlussfolgerung
Unsere Daten bestätigen den Nutzen von SSS für die anatomische Segmentierung von VS bei der LINAC-SRS/SRT-Behandlungsplanung und ergänzen Daten, die einen zusätzlichen Einsatz als nützliches FU-Instrument bei VS-Patienten rechtfertigen.



Similar content being viewed by others
Abbreviations
- CTC:
-
Common Terminology Criteria for Adverse Events
- FIESTA:
-
Fast imaging employing steady-state acquisition
- FU:
-
Follow-up
- GRE:
-
Gradient echo
- LINAC:
-
Linear accelerator
- SRS:
-
Stereotactic radiosurgery
- SRT:
-
Stereotactic radiotherapy
- SSS:
-
Steady-state sequences
- VS:
-
Vestibular schwannoma
References
Schmidt RF, Boghani Z, Choudhry OJ et al (2012) Incidental vestibular schwannomas: a review of prevalence, growth rate, and management challenges. Neurosurg Focus 33:E4
Kleijwegt M, Ho V, Visser O et al (2016) Real incidence of vestibular schwannoma? Estimations from a national registry. Otol Neurotol 37:1411–1417
Patel J, Vasan R, van Loveren H et al (2014) The changing face of acoustic neuroma management in the USA: analysis of the 1998 and 2008 patient surveys from the acoustic neuroma association. Br J Neurosurg 28:20–24
Stepanidis K, Kessel M, Caye-Thomasen P et al (2014) Socio-demographic distribution of vestibular schwannomas in Denmark. Acta Otolaryngol 134:551–556
Samii M, Matthies C (1997) Management of 1000 vestibular schwannomas (acoustic neuromas): hearing function in 1000 tumor resections. Neurosurgery 40:248–260 (discussion 260–242)
Lunsford LD, Niranjan A, Flickinger JC et al (2013) Radiosurgery of vestibular schwannomas: summary of experience in 829 cases. J Neurosurg. https://doi.org/10.3171/jns.2005.102.s_supplement.0195
Wolbers JG, Dallenga AH, Mendez Romero A et al (2013) What intervention is best practice for vestibular schwannomas? A systematic review of controlled studies. BMJ Open. https://doi.org/10.1136/bmjopen-2012-001345
Golfinos JG, Hill TC, Rokosh R et al (2016) A matched cohort comparison of clinical outcomes following microsurgical resection or stereotactic radiosurgery for patients with small- and medium-sized vestibular schwannomas. J Neurosurg 125:1472–1482
Klijn S, Verheul JB, Beute GN et al (2016) Gamma Knife radiosurgery for vestibular schwannomas: evaluation of tumor control and its predictors in a large patient cohort in The Netherlands. J Neurosurg 124:1619–1626
Persson O, Bartek J Jr., Shalom NB et al (2017) Stereotactic radiosurgery vs. fractionated radiotherapy for tumor control in vestibular schwannoma patients: a systematic review. Acta Neurochir (Wien) 159:1013–1021
Putz F, Muller J, Wimmer C et al (2017) Stereotactic radiotherapy of vestibular schwannoma: hearing preservation, vestibular function, and local control following primary and salvage radiotherapy. Strahlenther Onkol 193:200–212
Badakhshi H, Muellner S, Wiener E et al (2014) Image-guided stereotactic radiotherapy for patients with vestibular schwannoma. A clinical study. Strahlenther Onkol 190:533–537
Pollock BE (2008) Vestibular schwannoma management: an evidence-based comparison of stereotactic radiosurgery and microsurgical resection. Prog Neurol Surg 21:222–227
Combs SE, Engelhard C, Kopp C et al (2015) Long-term outcome after highly advanced single-dose or fractionated radiotherapy in patients with vestibular schwannomas—pooled results from 3 large German centers. Radiother Oncol 114:378–383
Schneider T, Chapiro J, Lin M et al (2016) 3D quantitative assessment of response to fractionated stereotactic radiotherapy and single-session stereotactic radiosurgery of vestibular schwannoma. Eur Radiol 26:849–857
Dunn IF, Bi WL, Mukundan S et al (2018) Congress of neurological surgeons systematic review and evidence-based guidelines on the role of imaging in the diagnosis and management of patients with vestibular schwannomas. Neurosurgery 82:E32–E34
Chavhan GB, Babyn PS, Jankharia BG et al (2008) Steady-state MR imaging sequences: physics, classification, and clinical applications. Radiographics 28:1147–1160
Scheffler K, Lehnhardt S (2003) Principles and applications of balanced SSFP techniques. Eur Radiol 13:2409–2418
Nitz WR (2002) Fast and ultrafast non-echo-planar MR imaging techniques. Eur Radiol 12:2866–2882
Park SH, Han PK, Choi SH (2015) Physiological and functional magnetic resonance imaging using balanced steady-state free precession. Korean J Radiol 16:550–559
Koos WT, Day JD, Matula C et al (1998) Neurotopographic considerations in the microsurgical treatment of small acoustic neurinomas. J Neurosurg 88:506–512
Gurgel RK, Jackler RK, Dobie RA et al (2012) A new standardized format for reporting hearing outcome in clinical trials. Otolaryngol Head Neck Surg 147:803–807
Hayhurst C, Zadeh G (2012) Tumor pseudoprogression following radiosurgery for vestibular schwannoma. Neuro-oncology 14:87–92
Yu CP, Cheung JY, Leung S et al (2000) Sequential volume mapping for confirmation of negative growth in vestibular schwannomas treated by gamma knife radiosurgery. J Neurosurg 93(Suppl 3):82–89
Henzel M, Hamm K, Sitter H et al (2009) Comparison of stereotactic radiosurgery and fractionated stereotactic radiotherapy of acoustic neurinomas according to 3‑D tumor volume shrinkage and quality of life. Strahlenther Onkol 185:567–573
Coelho DH, Tang Y, Suddarth B et al (2017) MRI surveillance of vestibular schwannomas without contrast enhancement: clinical and economic evaluation. Laryngoscope. https://doi.org/10.1002/lary.26589
van de Langenberg R, de Bondt BJ, Nelemans PJ et al (2011) Predictors of volumetric growth and auditory deterioration in vestibular schwannomas followed in a wait and scan policy. Otol Neurotol 32:338–344
van de Langenberg R, de Bondt BJ, Nelemans PJ et al (2009) Follow-up assessment of vestibular schwannomas: volume quantification versus two-dimensional measurements. Neuroradiology 51:517–524
Kondziolka D, Lunsford LD, McLaughlin MR et al (1998) Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med 339:1426–1433
Meijer OW, Weijmans EJ, Knol DL et al (2008) Tumor-volume changes after radiosurgery for vestibular schwannoma: implications for follow-up MR imaging protocol. AJNR Am J Neuroradiol 29:906–910
Linskey ME (2000) Stereotactic radiosurgery versus stereotactic radiotherapy for patients with vestibular schwannoma: a Leksell Gamma Knife Society 2000 debate. J Neurosurg 93(Suppl 3):90–95
del Valle R, Perez M, Ortiz J et al (2005) Stereotactic noninvasive volume measurement compared with geometric measurement for indications and evaluation of gamma knife treatment. J Neurosurg. https://doi.org/10.3171/jns.2005.102.s_supplement.0140
Luppino FS, Grooters E, de Bruine FT et al (2006) Volumetrical measurements in vestibular schwannoma, the influence of slice thickness and patient’s repositioning. Otol Neurotol 27:962–968
Jacob JT, Pollock BE, Carlson ML et al (2015) Stereotactic radiosurgery in the management of vestibular schwannoma and glomus jugulare: indications, techniques, and results. Otolaryngol Clin North Am 48:515–526
Nakamura H, Jokura H, Takahashi K et al (2000) Serial follow-up MR imaging after gamma knife radiosurgery for vestibular schwannoma. AJNR Am J Neuroradiol 21:1540–1546
Park CK, Kim DC, Park SH et al (2006) Microhemorrhage, a possible mechanism for cyst formation in vestibular schwannomas. J Neurosurg 105:576–580
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J.P. Sauer, T.M. Kinfe, B. Pintea, A. Schäfer, and J.P. Boström declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Sauer, J.P., Kinfe, T.M., Pintea, B. et al. The impact of MRI steady-state sequences as an additional assessment modality in vestibular schwannoma patients after LINAC stereotactic radiotherapy or radiosurgery. Strahlenther Onkol 194, 1103–1113 (2018). https://doi.org/10.1007/s00066-018-1317-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-018-1317-z
Keywords
- Vestibular schwannoma
- Linear accelerator
- Fractionated stereotactic radiotherapy
- Radiosurgery
- Steady-state sequences