Abstract
Background
Definition of gross tumor volume (GTV) in hepatocellular carcinoma (HCC) requires dedicated imaging in multiple contrast medium phases. The aim of this study was to evaluate the interobserver agreement (IOA) in gross tumor delineation of HCC in a multicenter panel.
Methods
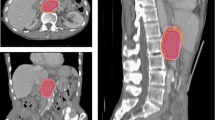
The analysis was performed within the “Stereotactic Radiotherapy” working group of the German Society for Radiation Oncology (DEGRO). The GTVs of three anonymized HCC cases were delineated by 16 physicians from nine centers using multiphasic CT scans. In the first case the tumor was well defined. The second patient had multifocal HCC (one conglomerate and one peripheral tumor) and was previously treated with transarterial chemoembolization (TACE). The peripheral lesion was adjacent to the previous TACE site. The last patient had an extensive HCC with a portal vein thrombosis (PVT) and an inhomogeneous liver parenchyma due to cirrhosis. The IOA was evaluated according to Landis and Koch.
Results
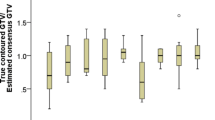
The IOA for the first case was excellent (kappa: 0.85); for the second case moderate (kappa: 0.48) for the peripheral tumor and substantial (kappa: 0.73) for the conglomerate. In the case of the peripheral tumor the inconsistency is most likely explained by the necrotic tumor cavity after TACE caudal to the viable tumor. In the last case the IOA was fair, with a kappa of 0.34, with significant heterogeneity concerning the borders of the tumor and the PVT.
Conclusion
The IOA was very good among the cases were the tumor was well defined. In complex cases, where the tumor did not show the typical characteristics, or in cases with Lipiodol (Guerbet, Paris, France) deposits, IOA agreement was compromised.
Zusammenfassung
Hintergrund
Die Definition des makroskopischen Tumorvolumens (GTV) bei hepatozellulären Karzinomen (HCC) erfordert eine dezidierte Bildgebung in mehreren Kontrastmittelphasen. Ziel dieser Studie war es, die Interobservervariabilität (IOA) bei der Konturierung von HCC-Läsionen durch ein multizentrisches Panel zu evaluieren.
Methoden
Die Analyse wurde von der Arbeitsgruppe Stereotaxie der deutschen Gesellschaft für Radioonkologie (DEGRO) durchgeführt. Die GTVs von 3 anonymisierten HCC-Patienten wurden von 16 Ärzten aus 9 Zentren mit Expertise in der Leberstereotaxie anhand multiphasischer Computertomogramme (CT) beurteilt. Beim ersten Patienten war der Tumor sehr gut abgrenzbar. Der zweite Patient hatte ein multilokuläres HCC (ein Konglomerat und ein peripherer Herd) und war zuvor mittels transarterieller Chemoembolisation (TACE) behandelt worden. Der periphere Herd lag direkt neben der TACE-Stelle. Der dritte Patient hatte einen schwer abzugrenzenden Tumor wegen ausgedehnten Leberinhomogenitäten bei ausgeprägter Leberzirrhose und einer begleitenden Pfortaderthrombose (PVT). Die IOA wurde nach Landis und Koch evaluiert.
Ergebnisse
Die IOA war beim ersten Patienten exzellent (Kappa: 0,85); im zweiten Fall moderat (Kappa: 0,48) für den peripheren Herd und substanziell (Kappa 0,73) für das Konglomerat. Beim peripheren Herd ist diese Inkonsistenz durch TACE kaudal des Tumors entstanden. Beim dritten Patienten war die IOA mit einem Kappa-Wert von 0,34 ausreichend aufgrund signifikanter Heterogenität hinsichtlich der genauen Tumorabgrenzung und dem PVT.
Diskussion
Die IOA war bei den gut abgrenzbaren Tumoren sehr gut. In komplexeren Fällen mit Perfussionsinhomogenitäten oder Lipiodolanreicherung durch Vortherapien war die IOA beeinträchtigt.




Similar content being viewed by others
References
Bruix J, Gores GJ, Mazzaferro V (2014) Hepatocellular carcinoma: clinical frontiers and perspectives. Gut 63(5):844–855. doi:10.1136/gutjnl-2013-306627
Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RK, Dinniwell RE, Kassam Z, Ringash J, Cummings B, Sykes J, Sherman M, Knox JJ, Dawson LA (2013) Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol 31(13):1631–1639. doi:10.1200/JCO.2012.44.1659
Sterzing F, Brunner TB, Ernst I, Baus WW, Greve B, Herfarth K, Guckenberger M (2014) Stereotactic body radiotherapy for liver tumors: principles and practical guidelines of the DEGRO Working Group on Stereotactic Radiotherapy. Strahlenther Onkol 190(10):872–881. doi:10.1007/s00066-014-0714-1
Verslype C, Rosmorduc O, Rougier P, Group EGW (2012) Hepatocellular carcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 23(Suppl 7):vii41–48. doi:10.1093/annonc/mds225
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174. doi:10.2307/2529310
Zijdenbos APDB, Margolin RA, Palmer AC (1994) Morphometric analysis of white matter lesions in MR images: method and validation. IEEE Trans Med Imaging 13(4):716–724
Kelemen ASG, Gerig G (1999) Elastic model-based segmentation of 3‑D neuroradiological data sets. IEEE Trans Med Imaging 18(10):828–839
Beddar AS, Briere TM, Balter P, Pan T, Tolani N, Ng C, Szklaruk J, Krishnan S (2008) 4D-CT imaging with synchronized intravenous contrast injection to improve delineation of liver tumors for treatment planning. Radiother Oncol 87(3):445–448. doi:10.1016/j.radonc.2007.12.009
Hong TS, Bosch WR, Krishnan S, Kim TK, Mamon HJ, Shyn P, Ben-Josef E, Seong J, Haddock MG, Cheng JC, Feng MU, Stephans KL, Roberge D, Crane C, Dawson LA (2014) Interobserver Variability in Target Definition for Hepatocellular Carcinoma With and Without Portal Vein Thrombus: Radiation Therapy Oncology Group Consensus Guidelines. Int J Radiat Oncol Biol Phys 89(4):804–813. doi:10.1016/j.ijrobp.2014.03.041
Kim YS, Kim JW, Yoon WS, Kang MK, Lee IJ, Kim TH, Kim JH, Lee HS, Park HC, Jang HS, Kay CS, Yoon SM, Kim MS, Seong J (2016) Interobserver variability in gross tumor volume delineation for hepatocellular carcinoma: Results of Korean Radiation Oncology Group 1207 study. Strahlenther Onkol 192(10):714–721. doi:10.1007/s00066-016-1028-2
Kanematsu M, Semelka RC, Leonardou P, Mastropasqua M, Lee JK (2003) Hepatocellular carcinoma of diffuse type: MR imaging findings and clinical manifestations. J Magn Reson Imaging 18(2):189–195. doi:10.1002/jmri.10336
Baron RL, Brancatelli G (2004) Computed tomographic imaging of hepatocellular carcinoma. Gastroenterology 127(5):133–S143. doi:10.1053/j.gastro.2004.09.027
European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer (2012) EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 56(4):908–943. doi:10.1016/j.jhep.2011.12.001
Tsurusaki M, Sofue K, Isoda H, Okada M, Kitajima K, Murakami T (2016) Comparison of gadoxetic acid-enhanced magnetic resonance imaging and contrast-enhanced computed tomography with histopathological examinations for the identification of hepatocellular carcinoma: a multicenter phase III study. J Gastroenterol 51(1):71–79. doi:10.1007/s00535-015-1097-5
Chou R, Cuevas C, Fu R, Devine B, Wasson N, Ginsburg A, Zakher B, Pappas M, Graham E, Sullivan SD (2015) Imaging techniques for the diagnosis of Hepatocellular carcinoma: a systematic review and meta-analysis. Ann Intern Med 162(10):697–711. doi:10.7326/M14-2509
Lee YJ, Lee JM, Lee JS, Lee HY, Park BH, Kim YH, Han JK, Choi BI (2015) Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging – a systematic review and meta-analysis. Radiology 275(1):97–109. doi:10.1148/radiol.14140690
Heimbach J, Kulik LM, Finn R, Sirlin CB, Abecassis M, Roberts LR, Zhu A, Murad MH, Marrero J (2017) Aasld guidelines for the treatment of hepatocellular carcinoma. Hepatology. doi:10.1002/hep.29086
Nestle U, Rischke HC, Eschmann SM, Holl G, Tosch M, Miederer M, Plotkin M, Essler M, Puskas C, Schimek-Jasch T, Duncker-Rohr V, Ruhl F, Leifert A, Mix M, Grosu AL, Konig J, Vach W (2015) Improved inter-observer agreement of an expert review panel in an oncology treatment trial – Insights from a structured interventional process. Eur J Cancer 51(17):2525–2533. doi:10.1016/j.ejca.2015.07.036
Schimek-Jasch T, Troost EG, Rucker G, Prokic V, Avlar M, Duncker-Rohr V, Mix M, Doll C, Grosu AL, Nestle U (2015) A teaching intervention in a contouring dummy run improved target volume delineation in locally advanced non-small cell lung cancer: Reducing the interobserver variability in multicentre clinical studies. Strahlenther Onkol 191(6):525–533. doi:10.1007/s00066-015-0812-8
Acknowledgements
The authors would like to thank G. Rückers for the statistical consulting.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
E. Gkika, S. Tanadini-Lang, S. Kirste, P.A. Holzner, H.P. Neeff, H.C. Rischke, T. Reese, F. Lohaus, M.N. Duma, K. Dieckmann, R. Semrau, M. Stockinger, D. Imhoff, N. Kremers, M.F. Häfner, N. Andratschke, U. Nestle, A.L. Grosu, M. Guckenberger, and T.B. Brunner declare that they have no competing interests.
Caption Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Gkika, E., Tanadini-Lang, S., Kirste, S. et al. Interobserver variability in target volume delineation of hepatocellular carcinoma. Strahlenther Onkol 193, 823–830 (2017). https://doi.org/10.1007/s00066-017-1177-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-017-1177-y