Abstract
Purpose
Recent studies have demonstrated low regional recurrence rates in early-stage breast cancer omitting axillary lymph node dissection (ALND) in patients who have positive nodes in sentinel lymph node dissection (SLND). This finding has triggered an active discussion about the effect of radiotherapy within this approach. The purpose of this study was to analyze the dose distribution in the axilla in standard tangential radiotherapy (SRT) for breast cancer and the effects on normal tissue exposure when anatomic level I–III axillary lymph node areas are included in the tangential radiotherapy field configuration.
Patients and methods
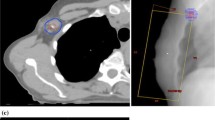
We prospectively analyzed the dosimetric treatment plans from 51 consecutive women with early-stage breast cancer undergoing radiotherapy. We compared and analyzed the SRT and the defined radiotherapy (DRT) methods for each patient. The clinical target volume (CTV) of SRT included the breast tissue without specific contouring of lymph node areas, whereas the CTV of DRT included the level I–III lymph node areas.
Results
We evaluated the dose given in SRT covering the axillary lymph node areas of level I–III as contoured in DRT. The mean VD95 % of the entire level I–III lymph node area in SRT was 50.28 % (range, 37.31–63.24 %), VD45 Gy was 70.1 % (54.8–85.4 %), and VD40 Gy was 83.5 % (72.3–94.8 %). A significant difference was observed between lung dose and heart toxicity in SRT vs. DRT. The V20 Gy and V30 Gy of the right and the left lung in DRT were significantly higher in DRT than in SRT (p < 0.001). The mean heart dose in SRT was significantly lower (3.93 vs. 4.72 Gy, p = 0.005).
Conclusion
We demonstrated a relevant dose exposure of the axilla in SRT that should substantially reduce local recurrences. Furthermore, we demonstrated a significant increase in lung and heart exposure when including the axillary lymph nodes regions in the tangential radiotherapy field set-up.
Zusammenfassung
Ziel
Aktuelle Studien zeigen niedrige regionäre Rezidive beim frühen Mammakarzinom, wenn trotz positiver Biopsie des Wächerlymphknotens („sentinel lymph node dissection“, SLND) keine Axilladissektion („axillary lymph node dissection“, ALND) angeschlossen wird. Diese Ergebnisse führten zu einer Diskussion über die Wertigkeit der adjuvanten tangentialen Strahlentherapie („standard tangential radiotherapy“, SRT) innerhalb dieses neuen Therapiekonzepts. Ziel der Studie ist es, die Dosisverteilung in der Axilla bei der SRT zu analysieren und den Effekt auf das umgebende Normalgewebe darzustellen, wenn der gesamte axilläre Lymphabfluß innerhalb einer definierten Radiotherapie (DRT) gezielt miterfasst wird.
Patienten und Methoden
Wir analysierten prospektiv 51 Therapiepläne von Patientinnen mit frühem Mammakarzinom, die eine Strahlentherapie erhielten. Bei jeder einzelnen Patientin wurden SRT und DRT verglichen und analysierten.
Ergebnisse
Wir evaluierten die Dosis im Bereich der axillären Lymphknotenregionen Level I–III bei SRT im Vergleich zu DRT. Das durchschnittliche VD95 % des gesamten Level I–III bei SRT betrug 50,28 % (Bereich 37,31–63,24 %), das VD45 Gy 70,1 % (Bereich 54,8–85,4 %) und das VD40 Gy 83,5 % (Bereich 72,3–94,8 %). V20 Gy und V30 Gy der rechten und linken Lunge bei DRT waren signifikant höher als bei SRT (p < 0,001) und die mittlere Herzbelastung bei SRT war signifikant niedriger (3,93 vs. 4,72 Gy, p = 0,005).
Schlussfolgerung
Wir zeigten eine relevante Dosis in der Axilla bei SRT, welche substantiell zur Senkung der regionären Rezidive beitragen sollte. Weiterhin fanden wir einen signifikanten Anstieg der Herz- und Lungenbelastung, wenn der gesamte axilläre Lymphabfluß im SRT-Setup gezielt miterfasst wird, auch wenn diese Befunde klinisch wenig relevant sind.




Similar content being viewed by others
References
Wernicke AG, Goodman RL, Turner BC et al (2011) A 10-year follow-up of treatment outcomes in patients with early stage breast cancer and clinically negative axillary nodes treated with tangential breast irradiation following sentinel lymph node dissection or axillary clearance. Breast Cancer Res Treat 125:893–902
Wong JS, Recht A, Beard CJ et al (1997) Treatment outcome after tangential radiation therapy without axillary dissection in patients with early-stage breast cancer and clinically negative axillary nodes. Int J Radiat Oncol Biol Phys 39:915–920
Hoskin PJ, Rajan B, Ebbs S et al (1992) Selective avoidance of lymphatic radiotherapy in the conservative management of early breast cancer. Radiother Oncol 25:83–88
Halverson KJ, Taylor ME, Perez CA et al (1993) Regional nodal management and patterns of failure following conservative surgery and radiation therapy for Stage I and II breast cancer. Int J Radiat Oncol BioI Phys 26:593–599
Kuznetsova M, Graybill J, Zusag TW et al (1995) Omission of axillary lymph node dissection in early-stage breast cancer: effect on treatment outcome. Radiology 197:507–510
Martelli G, Boracchi P, Ardoino I et al (2012) Axillary dissection versus no axillary dissection in older patients with T1N0 breast cancer: 15-year results of a randomized controlled trial. Ann Surg 256:920–924
Giuliano AE, Hunt KK, Ballman KV et al (2011) Axillary dissection vs. no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 305:569–575
Mansel RE, Fallowfield L, Kissin M et al (2006) Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst 98:599–609
Olson JA Jr, McCall LM, Beitsch P et al (2008) Impact of immediate versus delayed axillary node dissection on surgical outcomes in breast cancer patients with positive sentinel nodes: results from American College of Surgeons Oncology Group Trials Z0010 and Z0011. J Clin Oncol 26:3530–3535
Petrek JA, Heelan MC (1998) Incidence of breast carcinoma-related lymphedema. Cancer 83:2776–2781
Larson D, Weinstein M, Goldberg I et al (1986) Edema of the arm as a function of the extent of axillary surgery in patients with stage I–II carcinoma of the breast treated with primary radiotherapy. Int J Radiat Oncol Biol Phys 12:1575–1582
Galimberti V, Cole BF, Zurrida S et al, International Breast Cancer Study Group Trial 23–01 investigators (2013) Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23–01): a phase 3 randomised controlled trial. Lancet Oncol 14:297–305
Interdisziplinäre S3-Leitlinie für die Diagnostik, Therapie und Nachsorge des Mammakarzinoms, Langversion 3.0, Aktualisierung 2012, AWMF-Register-Nummer: 032–045OL. www.krebsgesellschaft.de. Accessed 9 May 2014
Scottish Intercollegiate Guidelines Network (SIGN). Treatment of primary breast cancer. Edinburgh: SIGN; 2013. (SIGN publication no. 134). [September 2013]. Available from URL: http://www.sign.ac.uk. Accessed 23 April 2014
NCCN (National Comprehensive Cancer Network®) Clinical Practice Guidelines in Oncology (NCCN Guidelines®), Breast Cancer, Version 3.2014, 04.01.14. www.NCCN.org. Accessed 9 May 2014
Sedlmayer F, Sautter-Bihl ML, Budach W et al, Breast Cancer Expert Panel of the German Society of Radiation Oncology (DEGRO) (2013) DEGRO practical guidelines: radiotherapy of breast cancer I: radiotherapy following breast conserving therapy for invasive breast cancer. Strahlenther Onkol 189:825–833
Orecchia R, Huscher A, Leonardi MC et al (2005) Irradiation with standard tangential breast fields in patients treated with conservative surgery and sentinel node biopsy: using a three-dimensional tool to evaluate the first level coverage of the axillary nodes. Br J Radiol 78:51–54
Reed DR, Lindsley SK, Mann GN et al (2005) Axillary lymph node dose with tangential breast irradiation. Int J Radiat Oncol Biol Phys 61:358–364
van Wely BJ, Teerenstra S, Schinagl DAX et al (2011) Systematic review of the effect of external beam radiation therapy to the breast on axillary recurrence after negative sentinel lymph node biopsy. Br J Surg 98:326–333
RTOG Breast Cancer Contouring Atlas. http://www.rtog.org/CoreLab/ContouringAtlases/BreastCancerAtlas.aspx, Accessed 15 March 2013
Nielsen MH, Berg M, Pedersen AN et al, Danish Breast Cancer Cooperative Group Radiotherapy Committee (2013) Delineation of target volumes and organs at risk in adjuvant radiotherapy of early breast cancer: national guidelines and contouring atlas by the Danish Breast Cancer Cooperative Group. Acta Oncol 52:703–710
Schlembach PJ, Buchholz TA, Ross MI et al (2001) Relationship of sentinel and axillary level I–II lymph nodes to tangential fields used in breast irradiation. Int J Radiat Oncol Biol Phys 51:671–678
Alço G, Iğdem SI, Ercan T et al (2010) Coverage of axillary lymph nodes with high tangential fields in breast radiotherapy. Br J Radiol 83:1072–1076
Belkacemi Y, Allab-Pan Q, Bigorie V et al (2013) The standard tangential fields used for breast irradiation do not allow optimal coverage and dose distribution in axillary levels I–II and the sentinel node area. Ann Oncol 24:2023–2028
Takeda A, Shigematsu N, Ikeda T et al (2004) Evaluation of novel modified tangential irradiation technique for breast cancer patients using dose-volume histograms. Int J Radiat Oncol Biol Phys 58:1280–1288
Mittendorf EA, Hunt KK, Boughey JC et al (2012) Incorporation of sentinel lymph node metastasis size into a nomogram predicting nonsentinel lymph node involvement in breast cancer patients with a positive sentinel lymph node. Ann Surg 255:109–115
Van Zee KJ, Manasseh DM, Bevilacqua JL et al (2003) A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol 10:1140–1151
Shahar KH, Hunt KK, Thames HD et al (2004) Factors predictive of having four or more positive axillary lymph nodes in patients with positive sentinel lymph nodes: implications for selection of radiation fields. Int J Radiat Oncol Biol Phys 59:1074–1079
Katz A, Smith BL, Golshan M et al (2008) Nomogram for the prediction of having four or more involved nodes for sentinel lymph node-positive breast cancer. J Clin Oncol 26:2093–2098
Chagpar AB, Scoggins CR, Martin RC 2nd et al, University of Louisville Breast Sentinel Lymph Node Study (2007) Predicting patients at low probability of requiring postmastectomy radiation therapy. Ann Surg Oncol 14:670–677
Dijkema IM, Hofman P, Raaijmakers CP et al (2004) Loco-regional conformal radiotherapy of the breast: delineation of the regional lymph node clinical target volumes in treatment position. Radiother Oncol 71:287–295
Reznik J, Cicchetti MG, Degaspe B et al (2005) Analysis of axillary coverage during tangential radiation therapy to the breast. Int J Radiat Oncol Biol Phys 61:163–168
Early Breast Cancer Trialists’ Collaborative Group (2000) Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Lancet 355:1757–1770
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Darby S, McGale P, Correa C et al (2011) Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 378:1707–1716
Rabinovitch R, Ballonoff A, Newman F et al (2008) Evaluation of breast sentinel lymph node coverage by standard radiation therapy fields. Int J Radiat Oncol Biol Phys 70:1468–1471
Darby SC, Ewertz M, McGale P et al (2013) Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 368:987–998
Dunst J (2013) Cardiac risks associated with adjuvant radiotherapy for breast cancer. Strahlenther Onkol 189:590–591
Graham MV, Purdy JA, Emami B et al (1999) Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 45:323–329
Marks LB, Bentzen SM, Deasy JO et al (2010) Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys 76:S70–S76
Haviland JS, Owen JR, Dewar JA et al (2013) The UK standardisation of breast radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-years follow-up results of two randomised controlled trials. Lancet Oncol 14:1086–1094
Compliance with ethical guidelines
Conflict of interest
M. Nitsche, N. Temme, M. Förster, M. Reible, and R. M. Hermann state that there are no conflicts of interest.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nitsche, M., Temme, N., Förster, M. et al. Tangential vs. defined radiotherapy in early breast cancer treatment without axillary lymph node dissection. Strahlenther Onkol 190, 715–721 (2014). https://doi.org/10.1007/s00066-014-0681-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-014-0681-6