Abstract
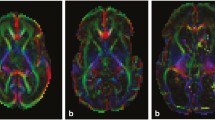
White matter lesions in hypoxic-ischemic encephalopathy (HIE) are considered to be the important substrate of frequent neurological consequences in preterm infants. The aim of the study was to analyze volumes and tractographic parameters of the cortico-ponto-cerebellar axis to assess alterations in the periventricular fiber system and crossroads, corticopontine and corticospinal pathways and prospective transsynaptic changes of the cerebellum.
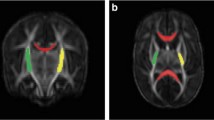
Term infants (control), premature infants without (normotypic) and with perinatal HIE (HIE) underwent brain magnetic resonance imaging at term-equivalent age (TEA) and at 2 years. Cerebrum, cerebellum, brainstem divisions and ventrodorsal compartments volumetric analysis were performed, as well as fractional anisotropy (FA) and apparent diffusion coefficient (ADC) of corticopontine, corticospinal pathways and middle cerebellar peduncles. Amiel-Tison scale at TEA and the Hempel test at 2 years were assessed.
Cerebellum, brainstem and its compartments volumes were decreased in normotypic and HIE groups at TEA, while at 2 years volumes were significantly reduced in the HIE group, accompanied by decreased volume and FA and increased ADC of corticopontine and corticospinal pathways. Negative association of the brainstem, cerebellum, mesencephalon, pons, corticopontine volumes and corticospinal pathway FA at TEA with the neurological score at 2 years. Cerebellum and pons volumes presented as potential prognostic indicators of neurological outcomes.
Our findings agree that these pathways, as a part of the periventricular fiber system and crossroads, exhibit lesion-induced reaction and vulnerability in HIE. Structural differences between normotypic and HIE group at the 2 years suggest a different developmental structural plasticity.








Similar content being viewed by others
References
Glass HC, Costarino AT, Stayer SA, Brett CM, Cladis F, Davis PJ. Outcomes for extremely premature infants. Anesth Analg. 2015;120:1337–51.
Miller SP, Ferriero DM, Leonard C, Piecuch R, Glidden DV, Partridge JC, Perez M, Mukherjee P, Vigneron DB, Barkovich AJ. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005;147:609–16.
Counsell SJ, Shen Y, Boardman JP, Larkman DJ, Kapellou O, Ward P, Allsop JM, Cowan FM, Hajnal JV, Edwards AD, Rutherford MA. Axial and radial diffusivity in preterm infants who have diffuse white matter changes on magnetic resonance imaging at term-equivalent age. Pediatrics. 2006;117:376–86.
Counsell SJ, Dyet LE, Larkman DJ, Nunes RG, Boardman JP, Allsop JM, Fitzpatrick J, Srinivasan L, Cowan FM, Hajnal JV, Rutherford MA, Edwards AD. Thalamo-cortical connectivity in children born preterm mapped using probabilistic magnetic resonance tractography. Neuroimage. 2007;34:896–904.
Voss W, Neubauer AP, Wachtendorf M, Verhey JF, Kattner E. Neurodevelopmental outcome in extremely low birth weight infants: what is the minimum age for reliable developmental prognosis? Acta Paediatr. 2007;96:342–7.
Mathur A, Inder T. Magnetic resonance imaging--insights into brain injury and outcomes in premature infants. J Commun Disord. 2009;42:248–55.
Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–24.
Glass HC, Shellhaas RA, Tsuchida TN, Chang T, Wusthoff CJ, Chu CJ, Cilio MR, Bonifacio SL, Massey SL, Abend NS, Soul JS; Neonatal Seizure Registry study group. Seizures in Preterm Neonates: A Multicenter Observational Cohort Study. Pediatr Neurol. 2017;72:19–24.
Orchinik LJ, Taylor HG, Espy KA, Minich N, Klein N, Sheffield T, Hack M. Cognitive outcomes for extremely preterm/extremely low birth weight children in kindergarten. J Int Neuropsychol Soc. 2011;17:1067–79.
Woodward LJ, Clark CA, Bora S, Inder TE. Neonatal white matter abnormalities an important predictor of neurocognitive outcome for very preterm children. PLoS One. 2012;7:e51879.
Kwon SH, Vasung L, Ment LR, Huppi PS. The role of neuroimaging in predicting neurodevelopmental outcomes of preterm neonates. Clin Perinatol. 2014;41:257–83.
Kostović I, Kostović-Srzentić M, Benjak V, Jovanov-Milošević N, Radoš M. Developmental dynamics of radial vulnerability in the cerebral compartments in preterm infants and neonates. Front Neurol. 2014;5:139.
Miller SP, Ferriero DM. From selective vulnerability to connectivity: insights from newborn brain imaging. Trends Neurosci. 2009;32:496–505.
Woodward LJ, Edgin JO, Thompson D, Inder TE. Object working memory deficits predicted by early brain injury and development in the preterm infant. Brain. 2005;128:2578–87.
Kostović I, Jovanov-Milošević N, Radoš M, Sedmak G, Benjak V, Kostović-Srzentić M, Vasung L, Čuljat M, Radoš M, Hüppi P, Judaš M. Perinatal and early postnatal reorganization of the subplate and related cellular compartments in the human cerebral wall as revealed by histological and MRI approaches. Brain Struct Funct. 2014;219:231–53.
Judaš M, Radoš M, Jovanov-Milošević N, Hrabac P, Kostović I. Structural, immunocytochemical, and MR imaging properties of periventricular crossroads of growing cortical pathways in preterm infants. AJNR Am J Neuroradiol. 2005;26:2671–84.
Vasung L, Huang H, Jovanov-Milošević N, Pletikos M, Mori S, Kostović I. Development of axonal pathways in the human fetal fronto-limbic brain: histochemical characterization and diffusion tensor imaging. J Anat. 2010;217:400–17.
Kidokoro H, Neil JJ, Inder TE. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am J Neuroradiol. 2013;34:2208–14.
Ment LR, Hirtz D, Hüppi PS. Imaging biomarkers of outcome in the developing preterm brain. Lancet Neurol. 2009;8:1042–55.
Volpe JJ. Cerebral white matter injury of the premature infant-more common than you think. Pediatrics. 2003;112:176–80.
Chao CP, Zaleski CG, Patton AC. Neonatal hypoxic-ischemic encephalopathy: multimodality imaging findings. Radiographics. 2006;26 Suppl 1:S159–72.
Eyre JA, Miller S, Clowry GJ, Conway EA, Watts C. Functional corticospinal projections are established prenatally in the human foetus permitting involvement in the development of spinal motor centres. Brain. 2000;123:51–64.
Eyre JA. Development and plasticity of the corticospinal system in man. Neural Plast. 2003;10:93–106.
Eyre JA. Corticospinal tract development and its plasticity after perinatal injury. Neurosci Biobehav Rev. 2007;31:1136–49.
Schmahmann JD, Pandya DN. Projections to the basis pontis from the superior temporal sulcus and superior temporal region in the rhesus monkey. J Comp Neurol. 1991;308:224–48.
Schmahmann JD, Pandya DN. Prefrontal cortex projections to the basilar pons in rhesus monkey: implications for the cerebellar contribution to higher function. Neurosci Lett. 1995;199:175–8.
Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–44.
Schmahmann JD, Rosene DL, Pandya DN. Motor projections to the basis pontis in rhesus monkey. J Comp Neurol. 2004;478:248–68.
Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 2006;7:511–22.
Kamali A, Kramer LA, Frye RE, Butler IJ, Hasan KM. Diffusion tensor tractography of the human brain cortico-ponto-cerebellar pathways: a quantitative preliminary study. J Magn Reson Imaging. 2010;32:809–17.
Palesi F, De Rinaldis A, Castellazzi G, Calamante F, Muhlert N, Chard D, Tournier JD, Magenes G, D’Angelo E, Gandini Wheeler-Kingshott CAM. Contralateral cortico-ponto-cerebellar pathways reconstruction in humans in vivo: implications for reciprocal cerebro-cerebellar structural connectivity in motor and non-motor areas. Sci Rep. 2017;7:12841.
Vasung L, Jovanov-Milošević N, Pletikos M, Mori S, Judaš M, Kostović I. Prominent periventricular fiber system related to ganglionic eminence and striatum in the human fetal cerebrum. Brain Struct Funct. 2011;215:237–53.
Vasung L, Raguz M, Kostovic I, Takahashi E. Spatiotemporal Relationship of Brain Pathways during Human Fetal Development Using High-Angular Resolution Diffusion MR Imaging and Histology. Front Neurosci. 2017;11:348.
Gao W, Lin W, Chen Y, Gerig G, Smith JK, Jewells V, Gilmore JH. Temporal and spatial development of axonal maturation and myelination of white matter in the developing brain. AJNR Am J Neuroradiol. 2009;30:290–6.
Gousias IS, Edwards AD, Rutherford MA, Counsell SJ, Hajnal JV, Rueckert D, Hammers A. Magnetic resonance imaging of the newborn brain: manual segmentation of labelled atlases in term-born and preterm infants. Neuroimage. 2012;62:1499–509.
Kostović Srzentić M, Raguž M, Ozretić D. Specific cognitive deficits in preschool age correlated with qualitative and quantitative MRI parameters in prematurely born children. Pediatr Neonatol. 2020;61:160–7.
Katušić A, Raguž M, Žunić Išasegi I. Brain tissue volumes at term-equivalent age are associated with early motor behavior in very preterm infants. Int J Dev Neurosci. 2020; doi: 10.1002/jdn.10039. Epub ahead of print.
Inder TE, Warfield SK, Wang H, Hüppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115:286–94.
de Kieviet JF, Zoetebier L, van Elburg RM, Vermeulen RJ, Oosterlaan J. Brain development of very preterm and very low-birthweight children in childhood and adolescence: a meta-analysis. Dev Med Child Neurol. 2012;54:313–23.
Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, Katz KH, Westerveld M, Sparrow S, Anderson AW, Duncan CC, Makuch RW, Gore JC, Ment LR. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284:1939–47.
Van Kooij BJ, Benders MJ, Anbeek P, Van Haastert IC, De Vries LS, Groenendaal F. Cerebellar volume and proton magnetic resonance spectroscopy at term, and neurodevelopment at 2 years of age in preterm infants. Dev Med Child Neurol. 2012;54:260–6.
Pierson CR, Folkerth RD, Billiards SS, Trachtenberg FL, Drinkwater ME, Volpe JJ, Kinney HC. Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathol. 2007;114:619–31.
Limperopoulos C, Chilingaryan G, Guizard N, Robertson RL, Du Plessis AJ. Cerebellar injury in the premature infant is associated with impaired growth of specific cerebral regions. Pediatr Res. 2010;68:145–50.
Tam EW, Ferriero DM, Xu D, Berman JI, Vigneron DB, Barkovich AJ, Miller SP. Cerebellar development in the preterm neonate: effect of supratentorial brain injury. Pediatr Res. 2009;66:102–6.
Tam EW, Miller SP, Studholme C, Chau V, Glidden D, Poskitt KJ, Ferriero DM, Barkovich AJ. Differential effects of intraventricular hemorrhage and white matter injury on preterm cerebellar growth. J Pediatr. 2011;158:366–71.
Limperopoulos C, Bassan H, Gauvreau K, Robertson RL Jr, Sullivan NR, Benson CB, Avery L, Stewart J, Soul JS, Ringer SA, Volpe JJ, duPlessis AJ. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics. 2007;120:584–93.
Limperopoulos C, Chilingaryan G, Sullivan N, Guizard N, Robertson RL, du Plessis AJ. Injury to the premature cerebellum: outcome is related to remote cortical development. Cereb Cortex. 2014;24:728–36.
Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–9.
Berman JI, Mukherjee P, Partridge SC, Miller SP, Ferriero DM, Barkovich AJ, Vigneron DB, Henry RG. Quantitative diffusion tensor MRI fiber tractography of sensorimotor white matter development in premature infants. Neuroimage. 2005;27:862–71.
Mori S, Wakana S, Nagae-Poetscher L, van Zijl PCM. MRI atlas of human white matter. Oxford, Elsevier, 2006.
Hermoye L, Saint-Martin C, Cosnard G, Lee SK, Kim J, Nassogne MC, Menten R, Clapuyt P, Donohue PK, Hua K, Wakana S, Jiang H, van Zijl PC, Mori S. Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. Neuroimage. 2006;29:493–504.
Barkovich AJ, Miller SP, Bartha A, Newton N, Hamrick SE, Mukherjee P, Glenn OA, Xu D, Partridge JC, Ferriero DM, Vigneron DB. MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. AJNR Am J Neuroradiol. 2006;27:533–47.
Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–32.
Huang H, Xue R, Zhang J, Ren T, Richards LJ, Yarowsky P, Miller MI, Mori S. Anatomical characterization of human fetal brain development with diffusion tensor magnetic resonance imaging. J Neurosci. 2009;29:4263–73.
Kidokoro H, Anderson PJ, Doyle LW, Neil JJ, Inder TE. High signal intensity on T2-weighted MR imaging at term-equivalent age in preterm infants does not predict 2-year neurodevelopmental outcomes. AJNR Am J Neuroradiol. 2011;32:2005–10.
Pandit AS, Ball G, Edwards AD, Counsell SJ. Diffusion magnetic resonance imaging in preterm brain injury. Neuroradiology. 2013;55 Suppl 2:65–95.
Counsell SJ, Rutherford MA, Cowan FM, Edwards AD. Magnetic resonance imaging of preterm brain injury. Arch Dis Child Fetal Neonatal Ed. 2003;88:F269–74.
Counsell SJ, Boardman JP. Differential brain growth in the infant born preterm: current knowledge and future developments from brain imaging. Semin Fetal Neonatal Med. 2005;10:403–10.
Logitharajah P, Rutherford MA, Cowan FM. Hypoxic-ischemic encephalopathy in preterm infants: antecedent factors, brain imaging, and outcome. Pediatr Res. 2009;66:222–9.
Mai J, Majtanik M, Paxinos G. Atlas of the human brain. 4th ed. San Diego: Academic Press, Elsevier; 2015.
Van Hecke W, Emsell L, Sunaert S. Diffusion Tensor Imaging. New York: Springer; 2016.
Amiel-Tison C. Update of the Amiel-Tison neurologic assessment for the term neonate or at 40 weeks corrected age. Pediatr Neurol. 2002;27:196–212.
Hempel MS. Neurological development during toddling age in normal children and children at risk of developmental disorders. Early Hum Dev. 1993;34:47–57.
Conover WJ. Practical nonparametric statistics. New York: John Wiley & Sons; 1999.
Žunić Išasegi I, Radoš M, Krsnik Ž, Radoš M, Benjak V, Kostović I. Interactive histogenesis of axonal strata and proliferative zones in the human fetal cerebral wall. Brain Struct Funct. 2018;223:3919–43.
Counsell SJ, Allsop JM, Harrison MC, Larkman DJ, Kennea NL, Kapellou O, Cowan FM, Hajnal JV, Edwards AD, Rutherford MA. Diffusion-weighted imaging of the brain in preterm infants with focal and diffuse white matter abnormality. Pediatrics. 2003;112:1–7.
Hüppi PS, Murphy B, Maier SE, Zientara GP, Inder TE, Barnes PD, Kikinis R, Jolesz FA, Volpe JJ. Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics. 2001;107:455–60.
Valkama AM, Tolonen EU, Kerttul LI, Pääkkö EL, Vainionpää LK, Koivist ME. Brainstem size and function at term age in relation to later neurosensory disability in high-risk, preterm infants. Acta Paediatr. 2001;90:909–15.
Knickmeyer RC, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, Hamer RM, Lin W, Gerig G, Gilmore JH. A structural MRI study of human brain development from birth to 2 years. J Neurosci. 2008;28:12176–82.
Luo L, O’Leary DD. Axon retraction and degeneration in development and disease. Annu Rev Neurosci. 2005;28:127–56.
O’Leary DD, Terashima T. Cortical axons branch to multiple subcortical targets by interstitial axon budding: implications for target recognition and “waiting periods”. Neuron. 1988;1:901–10.
Rakic P, Lombroso PJ. Development of the cerebral cortex: I. Forming the cortical structure. J Am Acad Child Adolesc Psychiatry. 1998;37:116–7.
Rakic P. Images in neuroscience. Brain development, VI: radial migration and cortical evolution. Am J Psychiatry. 1998;155:1150–1.
Kostovic I, Zecevic N. Development of the human cerebellar cortex: change in cholinesterase activity during prenatal period. Bulletin de l’Académie Serbe des Sciences et des Arts. Classe des sciences médicales. 1984;87:1–8.
Sidman RL, Rakic P. Neuronal migration, with special reference to developing human brain: a review. Brain Res. 1973;62:1–35.
Ramon Y Cajal S, May MR. Degeneration and regeneration of the nervous system. London: Oxford University Press; 1928.
Smith MC. Histological findings after hemicerebellectomy in man: anterograde, retrograde and transneuronal degeneration. Brain Res. 1975;95:423–42.
Dinkin M. Trans-synaptic Retrograde Degeneration in the Human Visual System: Slow, Silent, and Real. Curr Neurol Neurosci Rep. 2017;17:16.
Volpe JJ. Neurology of the newborn. Philadelphia: Saunders; 2001.
Goldman-Rakic PS. Prenatal formation of cortical input and development of cytoarchitectonic compartments in the neostriatum of the rhesus monkey. J Neurosci. 1981;1:721–35.
Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12:719–43.
Staudt M. (Re-)organization of the developing human brain following periventricular white matter lesions. Neurosci Biobehav Rev. 2007;31:1150–6.
Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18:383–8.
Ribeiro Gomes AR, Olivier E, Killackey HP, Giroud P, Berland M, Knoblauch K, Dehay C, Kennedy H. Refinement of the Primate Corticospinal Pathway During Prenatal Development. Cereb Cortex. 2020;30:656–71.
Miller SP, Vigneron DB, Henry RG, Bohland MA, Ceppi-Cozzio C, Hoffman C, Newton N, Partridge JC, Ferriero DM, Barkovich AJ. Serial quantitative diffusion tensor MRI of the premature brain: development in newborns with and without injury. J Magn Reson Imaging. 2002;16:621–32.
Beck E. The origin, course and termination of the prefronto-pontine tract in the human brain. Brain. 1950;73:368–91.
Makropoulos A, Counsell SJ, Rueckert D. A review on automatic fetal and neonatal brain MRI segmentation. Neuroimage. 2018;170:231–48.
Weisenfeld NI, Warfield SK. Automatic segmentation of newborn brain MRI. Neuroimage. 2009;47:564–72.
Funding
The research was funded by Croatian Science Foundation projects CSF-IP-09-2014-4517, CSF-IP-2013-11-7379, CSF-IP-09-2014-7406 and CSF-DOK-10-2015. This work was supported in part by the “Research Cooperability” Program of the Croatian Science Foundation funded by the European Union from the European Social Fund under the Operational Programme Efficient Human Resources 2014–2020 PSZ-2019-02-4710. It was also co-financed by the Scientific Centre of Excellence for Basic, Clinical and Translational Neuroscience (project “Experimental and clinical research of hypoxic-ischemic damage in perinatal and adult brain”; GA KK01.1.1.01.0007 funded by the European Union through the European Regional Development Fund).
Author information
Authors and Affiliations
Contributions
MaR designed the study, conducted volumetric and tractographic analysis, wrote the paper and interpreted the results. MiR designed the study, performed all MRI scans, contributed to data analysis and interpretation. MKS and NK contributed to statistical data analysis and interpretation of results. IZI contributed in volumetric analysis and interpretation of results. VB and TC helped with the clinical part of neurological examination of prematurely born children and with collecting clinical data. MV contributed to data analysis. IK designed the study, wrote the paper, and interpreted the results. All authors approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
M. Raguž, M. Radoš, M. Kostović Srzetić, N. Kovačić, I. Žunić Išasegi, V. Benjak, T. Ćaleta, M. Vukšić and I. Kostović declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical standards
This study was carried out in accordance with the recommendations of ethics board of the University of Zagreb, School of Medicine. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Institutional Review Board of the University of Zagreb, School of Medicine.
Additional information
Availability of Data and Material
The datasets generated for this study are available on request to the corresponding author.
Rights and permissions
About this article
Cite this article
Raguž, M., Radoš, M., Kostović Srzetić, M. et al. Structural Changes in the Cortico-Ponto-Cerebellar Axis at Birth are Associated with Abnormal Neurological Outcomes in Childhood. Clin Neuroradiol 31, 1005–1020 (2021). https://doi.org/10.1007/s00062-021-01017-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-021-01017-1