Abstract
Purpose
To investigate the cerebral macrovascular changes as well as the relationship of large vessels and cerebral blood flow (CBF) in mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS) using magnetic resonance angiography (MRA) and arterial spin labeling (ASL) perfusion MR imaging (MRI).
Methods
A total of 20 patients diagnosed with MELAS (12 males, 8 females; mean age, 23.3 years) underwent conventional MRI, time-of-flight (TOF) MRA and three dimensional ASL. Follow-up scans were performed in 10 patients. The changes of cerebral arteries and branches on MRA images from both acute and recovery patients were independently evaluated by two radiologists. Lesion distribution and CBF were observed on the integrated maps of MRA and ASL.
Results
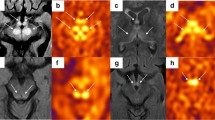
In 14 patients with clinical onsets, increased CBF was observed in all stroke-like lesions. Dilations of a single artery (four middle cerebral arteries, two posterior cerebral arteries) were found in six patients. Dilations of multiple arteries (two anterior cerebral arteries, seven middle cerebral arteries, six posterior cerebral arteries) were found in seven patients. Normal angiography was shown in one acute patient. Cortical terminal branches feeding the lesion areas were more obviously dilated than the main trunks. The dilated vessels returned to normal on follow-up scans concurrently with decreased CBF in nine patients who were resuscitated from episode attacks. Vasodilation was even seen in one preclinical patient who suffered a recurrent episode 50 days later.
Conclusion
Reversible dilation of cerebral macrovascular changes could be a new feature of MELAS and a presumed reason for fluctuant CBF. It would shed new light on the mitochondrial angiopathy.



Similar content being viewed by others
References
Yatsuga S, Povalko N, Nishioka J, Katayama K, Kakimoto N, Matsuishi T, Kakuma T, Koga Y; Taro Matsuoka for MELAS Study Group in Japan. MELAS: a nationwide prospective cohort study of 96 patients in Japan. Biochim Biophys Acta. 2012;1820:619-24.
El-Hattab AW, Adesina AM, Jones J, Scaglia F. MELAS syndrome: clinical manifestations, pathogenesis, and treatment options. Mol Genet Metab. 2015;116:4-12.
Tzoulis C, Bindoff LA. Acute mitochondrial encephalopathy reflects neuronal energy failure irrespective of which genome the genetic defect affects. Brain. 2012;135(Pt 12):3627–34.
Ito H, Mori K, Harada M, Minato M, Naito E, Takeuchi M, Kuroda Y, Kagami S. Serial brain imaging analysis of stroke-like episodes in MELAS. Brain Dev. 2008;30:483-8.
Li R, Xiao HF, Lyu JH, Wang D JJ, Ma L, Lou X. Differential diagnosis of mitochondrial encephalopathy with lactic acidosis and stroke-like episodes (MELAS) and ischemic stroke using 3D pseudocontinuous arterial spin labeling. J Magn Reson Imaging. 2017;45:199-206.
Bersano A, Markus HS, Quaglini S, Arbustini E, Lanfranconi S, Micieli G, Boncoraglio GB, Taroni F, Gellera C, Baratta S, Penco S, Mosca L, Grasso M, Carrera P, Ferrari M, Cereda C, Grieco G, Corti S, Ronchi D, Bassi MT, Obici L, Parati EA, Pezzini A, De Lodovici ML, Verrengia EP, Bono G, Mazucchelli F, Zarcone D, Calloni MV, Perrone P, Bordo BM, Colombo A, Padovani A, Cavallini A, Beretta S, Ferrarese C, Motto C, Agostoni E, Molini G, Sasanelli F, Corato M, Marcheselli S, Sessa M, Comi G, Checcarelli N, Guidotti M, Uccellini D, Capitani E, Tancredi L, Arnaboldi M, Incorvaia B, Tadeo CS, Fusi L, Grampa G, Merlini G, Trobia N, Comi GP, Braga M, Vitali P, Baron P, Grond-Ginsbach C, Candelise L; Lombardia GENS Group*. Clinical pregenetic screening for stroke monogenic diseases: results from lLombardia GENS Registry. Stroke. 2016;47:1702-9.
Tatlisumak T, Putaala J, Innilä M, Enzinger C, Metso TM, Curtze S, von Sarnowski B, Amaral-Silva A, Jungehulsing GJ, Tanislav C, Thijs V, Rolfs A, Norrving B, Fazekas F, Suomalainen A, Kolodny EH. Frequency of MELAS main mutation in a phenotype-targeted young ischemic stroke patient population. J Neurol. 2016;263:257-62.
Koopman WJ, Willems PH, Smeitink JA. Monogenic mitochondrial disorders. N Engl J Med. 2012;366:1132-41.
Sarnat HB, Flores-Sarnat L, Casey R, Scott P, Khan A. Endothelial ultrastructural alterations of intramuscular capillaries in infantile mitochondrial cytopathies: “mitochondrial angiopathy”. Neuropathology. 2012;32:617-27.
Lax NZ, Pienaar IS, Reeve AK, Hepplewhite PD, Jaros E, Taylor RW, Kalaria RN, Turnbull DM. Microangiopathy in the cerebellum of patients with mitochondrial DNA disease. Brain. 2012;135(Pt 6):1736-50.
Kim JH, Lim MK, Jeon TY, Rha JH, Eo H, Yoo SY, Shu CH. Diffusion and perfusion characteristics of MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episode) in thirteen patients. Korean J Radiol. 2011;12:15-24.
Rodan LH, Poublanc J, Fisher JA, Sobczyk O, Wong T, Hlasny E, Mikulis D, Tein I. Cerebral hyperperfusion and decreased cerebrovascular reactivity correlate with neurologic disease severity in MELAS. Mitochondrion. 2015;22:66–74.
Yoshida T, Ouchi A, Miura D, Shimoji K, Kinjo K, Sueyoshi T, Jonosono M, Rajput V. MELAS and reversible vasoconstriction of the major cerebral arteries. Intern Med. 2013;52:1389-92.
Noguchi A, Shoji Y, Matsumori M, Komatsu K, Takada G. Stroke-like episode involving a cerebral artery in a patient with MELAS. Pediatr Neurol. 2005;33:70-1.
Minobe S, Matsuda A, Mitsuhashi T, Ishikawa M, Nishimura Y, Shibata K, Ito E, Goto Y, Nakaoka T, Sakura H. Vasodilatation of multiple cerebral arteries in early stage of stroke-like episode with MELAS. J Clin Neurosci. 2015;22:407-8.
Ikawa M, Yoneda M, Muramatsu T, Matsunaga A, Tsujikawa T, Yamamoto T, Kosaka N, Kinoshita K, Yamamura O, Hamano T, Nakamoto Y, Kimura H. Detection of preclinically latent hyperperfusion due to stroke-like episodes by arterial spin-labeling perfusion MRI in MELAS patients. Mitochondrion. 2013;13:676-80.
Finsterer J, Zarrouk-Mahjoub S. Macroangiopathy is a typical phenotypic manifestation of MELAS. Metab Brain Dis. 2017;32:977-9.
Finsterer J, Zarrouk-Mahjoub S. Mitochondrial vasculopathy. World J Cardiol. 2016;8:333-9.
Iizuka T, Sakai F, Suzuki N, Hata T, Tsukahara S, Fukuda M, Takiyama Y. Neuronal hyperexcitability in stroke-like episodes of melas syndrome. Neurology. 2002;59:816-24.
Yoneda M, Ikawa M, Arakawa K, Kudo T, Kimura H, Fujibayashi Y, Okazawa H. In vivo functional brain imaging and a therapeutic trial of L‑arginine in MELAS patients. Biochim Biophys Acta. 2012;1820:615-8.
Tay SH, Nordli DR Jr, Bonilla E, Null E, Monaco S, Hirano M, DiMauro S. Aortic rupture in mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes. Arch Neurol. 2006;63:281-3.
Iizuka T, Goto Y, Miyakawa S, Sato M, Wang Z, Suzuki K, Hamada J, Kurata A, Sakai F. Progressive carotid artery stenosis with a novel tRNA phenylalanine mitochondrial DNA mutation. J Neurol Sci. 2009;278:35-40.
Mancuso M, Montano V, Orsucci D, Peverelli L, Caputi L, Gambaro P, Siciliano G, Lamperti C. Mitochondrial vasculopathy due to the m.3243A > G mutation is not restricted to the carotid artery. Mol Genet Metab Rep. 2016;9:12-4.
Finsterer JBA. Dilative arteriopathy and leucencephalopathy as manifestations of a neurometabolic disease. Open Neurol J. 2015;26:28-31.
Zhu K, Li S, Chen H, Wang Y, Yu M, Wang H, Zhao W, Cao Y. Late onset MELAS with m.3243A > G mutation and its association with aneurysm formation. Metab Brain Dis. 2017;32:1069-72.
Bizeau A, Gilbert G, Bernier M, Huynh MT, Bocti C, Descoteaux M, Whittingstall K. Stimulus-evoked changes in cerebral vessel diameter: a study in healthy humans. J Cereb Blood Flow Metab. 2017 Jan 1 [Epub ahead of print]
Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8-17.
Muñoz MF, Puebla M, Figueroa XF. Control of the neurovascular coupling by nitric oxide-dependent regulation of astrocytic Ca(2+) signaling. Front Cell Neurosci. 2015;9:59.
Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci. 2017;18:419-34.
Gropen TI, Prohovnik I, Tatemichi TK, Hirano M. Cerebral hyperemia in MELAS. Stroke. 1994;25(9):1873–6.
Li Y, Lin J, Sun C, Zhao C, Li H. Increased cerebral blood flow as a predictor of episodes in MELAS using multimodal MRI. J Magn Reson Imaging. 2017;46:915–8.
Acknowledgements
We would like to thank all patients who participated in this study. We also acknowledge the grants from the National Natural Science Foundation of China (No. 81301203, 81401035) which supported this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Y. Li, W. Xu, C. Sun, J. Lin, J. Qu, J. Cao, H. Li and L. Yang declare that they have no competing interests.
Ethical standards
All investigations performed on humans in this study were carried out in accordance with national law and the Helsinki Declaration from 1964 (in its current revised form). This study was approved by the ethics committee of our hospital. Informed consent was obtained from each patient.
Additional information
Dr. Weixingzi Xu and Dr. Chong Sun both participated in the project design and revised the manuscript. In addition, Dr. Xu analyzed the imaging data and Dr. Sun managed the patients.
Caption Electronic Supplementary Material
62_2018_662_MOESM1_ESM.pdf
ESM-Fig. 1 MRA images (inferior-superior view) of each patient with MELAS at the initial MR scans; Fig. 2 MRA images (inferior-superior view) of ten patients with MELAS at the follow-up MR scans
Rights and permissions
About this article
Cite this article
Li, Y., Xu, W., Sun, C. et al. Reversible Dilation of Cerebral Macrovascular Changes in MELAS Episodes. Clin Neuroradiol 29, 321–329 (2019). https://doi.org/10.1007/s00062-018-0662-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-018-0662-8