Abstract
Background
Diabetes mellitus is known to be associated with worse clinical outcomes in patients with coronary artery disease (CAD) undergoing percutaneous coronary interventions (PCI) with drug-eluting stents (DES). Defining the optimal duration of dual antiplatelet therapy (DAPT) after DES implantation is still under debate. The objective of this subgroup analysis of the all-comers ISAR 2000 registry was to assess the safety and efficacy of a short DAPT (<6 month) versus a longer DAPT (>6 month) in patients with diabetes electively treated with the polymer-free sirolimus-coated ultrathin strut drug-eluting stent (PF-SES).
Methods
Patients who received the PF-SES were investigated in a multicenter all-comers observational study. The primary endpoint was the 9‑month target lesion revascularization (TLR) rate, whereas secondary endpoints included the 9‑month major adverse cardiac event (MACE) and procedural success rates.
Results
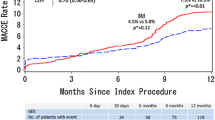
In all, 167 patients were treated with DAPT for ≤6 months (S-DAPT group) and 350 patients underwent DAPT treatment for 12 months (L-DAPT group). There was no significant difference in the overall MACE rate (4.6% vs. 3.1%, p = 0.441), the 9‑month accumulated stent thrombosis rates (0.8% vs. 0.3%, p = 0.51), or the accumulated rate of bleeding complications (5.3% vs. 3.4%, p = 0.341).
Conclusion
PF-SES are safe and effective in daily clinical routine with low rates of TLR and MACE in patients with diabetes and stable disease. Our data suggest that extending the duration of DAPT beyond 6 months does not improve MACE or TLR at 9 months in patients with stable CAD (ClinicalTrials.gov Identifier NCT02629575).
Zusammenfassung
Hintergrund
Patienten mit Diabetes, die sich einer perkutanten Koronarintervention (PCI) mit medikamentenbeschichteten Stents („drug-eluting stents“, DES) unterziehen, haben im Vergleich zu Nichtdiabetikern eine schlechtere Prognose. Ein möglicher Faktor könnte die Notwendigkeit einer verlängerten dualen Thrombozytenaggregationshemmung (DAPT) darstellen. Die optimale Dauer der DAPT nach DES-Implantation wird derzeit kontrovers diskutiert. Es wurde eine Subgruppenanalyse des ISAR-2000-Registers durchgeführt, um zu untersuchen, ob eine verkürzte DAPT (<6 Monate) im Vergleich zu einer längeren DAPT (>6 Monate) bei Diabetikern sicher und wirksam ist, bei denen eine elektive PCI mit einem polymerfreien sirolimusfreisetzenden ultradünnen Stent („polymer-free sirolimus coated ultrathin strut drug eluting stent“, PF-SES) durchgeführt wurde.
Methoden
Die Daten der mit einem PF-SES behandelten Patienten wurden im Rahmen einer multizentrischen Beobachtungsstudie anhand eines Registers in Asien und Europa analysiert. Der primäre Endpunkt war die 9‑monatige Revaskularisationsrate („target lesion revascularization“, TLR); sekundäre Endpunkte beinhalteten die Rate schwerer kardiovaskulärer Ereignisse („major adverse cardiac events“, MACE) und die Rate des prozeduralen Erfolgs.
Ergebnisse
Eine verkürzte DAPT erhielten 167 Patienten (<6 Monate; S‑DAPT-Gruppe), und 350 Patienten wurden mit einer längeren DAPT (>6 Monate; L‑DAPT-Gruppe) behandelt. Es zeigte sich kein signifikanter Unterschied hinsichtlich der MACE-Rate (4,6 % S‑DAPT; 3,1 % L‑DAPT; p = 0,441), der 9‑monatigen Stentthromboserate (0,8 % S‑DAPT; 0,3 % L‑DAPT; p = 0,51) oder der kumulativen Rate an Blutungskomplikationen nach 9 Monaten (5,3 % S‑DAPT; 3,4 % L‑DAPT; p = 0,341).
Schlussfolgerung
Die vorliegenden Daten zeigen, dass der Einsatz von PF-SES in der klinischen Routine bei Patienten mit Diabetes mellitus und stabiler koronare Herzerkrankung sicher und wirksam ist. Diese Daten weisen darauf hin, dass eine DAPT, die länger als 6 Monate dauert, zu keiner Verbesserung der MACE- oder TLR-Rate nach 9 Monaten führt (ClinicalTrials.gov Identifier NCT02629575).
Similar content being viewed by others
References
Hlatky MA, Boothroyd DB, Bravata DM, Boersma E, Booth J, Brooks MM et al (2009) Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet 373(9670):1190–1197
Hong SJ, Kim MH, Ahn TH, Ahn YK, Bae JH, Shim WJ et al (2006) Multiple predictors of coronary restenosis after drug-eluting stent implantation in patients with diabetes. Heart 92(8):1119–1124
Wei CC, Shyu KG, Cheng JJ, Lo HM, Chiu CZ (2016) Diabetes and Adverse Cardiovascular Outcomes in Patients with Acute Coronary Syndrome – Data from Taiwan’s Acute Coronary Syndrome Full Spectrum Data Registry. Acta Cardiol Sin 32(1):31–38
Iijima R, Byrne RA, Dibra A, Ndrepepa G, Spaulding C, Laarman GJ et al (2009) Drug-eluting stents versus bare-metal stents in diabetic patients with ST-segment elevation acute myocardial infarction: a pooled analysis of individual patient data from seven randomized trials. Rev Esp Cardiol 62(4):354–364
De Luca G, Dirksen MT, Spaulding C, Kelbaek H, Schalij M, Thuesen L et al (2013) Impact of diabetes on long-term outcome after primary angioplasty: insights from the DESERT cooperation. Diabetes Care 36(4):1020–1025
Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G et al (2005) Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 293(17):2126–2130
El-Hayek G, Messerli F, Bangalore S, Hong MK, Herzog E, Benjo A et al (2014) Meta-analysis of randomized clinical trials comparing short-term versus long-term dual antiplatelet therapy following drug-eluting stents. Am J Cardiol 114(2):236–242
Grodzinsky A, Arnold SV, Wang TY, Sharma P, Gosch K, Jones PG et al (2016) Bleeding risk following percutaneous coronary intervention in patients with diabetes prescribed dual anti-platelet therapy. Am Heart J 182:111–118
Montalescot G, Wiviott SD, Braunwald E, Murphy SA, Gibson CM, McCabe CH et al (2009) Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Lancet 373(9665):723–731
Spencer FA, Prasad M, Vandvik PO, Chetan D, Zhou Q, Guyatt G (2015) Longer- Versus Shorter-Duration Dual-Antiplatelet Therapy After Drug-Eluting Stent Placement: A Systematic Review and Meta-analysis. Ann Intern Med 163(2):118–126
Raber L, Magro M, Stefanini GG, Kalesan B, van Domburg RT, Onuma Y et al (2012) Very late coronary stent thrombosis of a newer-generation everolimus-eluting stent compared with early-generation drug-eluting stents: a prospective cohort study. Circulation 125(9):1110–1121
Ziada KM, Abdel-Latif A, Charnigo R, Moliterno DJ (2016) Safety of an abbreviated duration of dual antiplatelet therapy (〈/=6 months) following second-generation drug-eluting stents for coronary artery disease: A systematic review and meta-analysis of randomized trials. Catheter Cardiovasc Interv 87(4):722–732
Krackhardt F, Noutsias M, Tschope C, Kherad B (2017) DCB meets DES. Herz 42(7):696–697
Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA et al (2007) Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 115(17):2344–2351
Leschke M, Waliszewski M, Pons M, Champin S, Nait Saidi L, Mok Heang T et al (2016) Thin strut bare metal stents in patients with atrial fibrillation: Is there still a need for BMS? Catheter Cardiovasc Interv 88(3):358–366
Massberg S, Byrne RA, Kastrati A, Schulz S, Pache J, Hausleiter J et al (2011) Polymer-free sirolimus- and probucol-eluting versus new generation zotarolimus-eluting stents in coronary artery disease: the Intracoronary Stenting and Angiographic Results: Test Efficacy of Sirolimus- and Probucol-Eluting versus Zotarolimus-eluting Stents (ISAR-TEST 5) trial. Circulation 124(5):624–632
Kufner S, Sorges J, Mehilli J, Cassese S, Repp J, Wiebe J, et al. Randomized Trial of Polymer-Free Sirolimus- and Probucol-Eluting Stents Versus Durable Polymer Zotarolimus-Eluting Stents (2016) 5‑Year Results of the ISAR-TEST-5 Trial. Jacc Cardiovasc Interv 9(8):784–792
Colleran R, Kufner S, Harada Y, Giacoppo D, Cassese S, Repp J et al (2016) Five-year follow-up of polymer-free sirolimus- and probucol-eluting stents versus new generation zotarolimus-eluting stents in patients presenting with st-elevation myocardial infarction. Catheter Cardiovasc Interv 89(3):367–374
Harada Y, Colleran R, Kufner S, Giacoppo D, Rheude T, Michel J et al (2016) Five-year clinical outcomes in patients with diabetes mellitus treated with polymer-free sirolimus- and probucol-eluting stents versus second-generation zotarolimus-eluting stents: a subgroup analysis of a randomized controlled trial. Cardiovasc Diabetol 15(1):124
Kolh P, Windecker S (2014) ESC/EACTS myocardial revascularization guidelines 2014. Eur Heart J 35(46:3235–3236
Wohrle J, Zadura M, Mobius-Winkler S, Leschke M, Opitz C, Ahmed W et al (2012) SeQuentPlease World Wide Registry: clinical results of SeQuent please paclitaxel-coated balloon angioplasty in a large-scale, prospective registry study. J Am Coll Cardiol 60(18):1733–1738
Liu C, Liu M, Chen D, Liu H, Jiang Q, Lu J et al (2015) Effectiveness of prolonged clopidogrel-based dual antiplatelet therapy after drug-eluting stent implantation: Evidence-based meta-analysis. Herz 40(5):795–802
Chen CF, Chen B, Zhu J, Xu YZ (2015) Antithrombotic therapy after percutaneous coronary intervention in patients requiring oral anticoagulant treatment. A meta-analysis. Herz 40(8):1070–1083
Villablanca PA, Massera D, Mathew V, Bangalore S, Christia P, Perez I et al (2016) Outcomes of 〈/=6-month versus 12-month dual antiplatelet therapy after drug-eluting stent implantation: A meta-analysis and meta-regression. Medicine (Baltimore) 95(52):e5819
Schulz S, Schuster T, Mehilli J, Byrne RA, Ellert J, Massberg S et al (2009) Stent thrombosis after drug-eluting stent implantation: incidence, timing, and relation to discontinuation of clopidogrel therapy over a 4-year period. Eur Heart J 30(22):2714–2721
Gwon HC, Hahn JY, Park KW, Song YB, Chae IH, Lim DS et al (2012) Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation 125(3):505–513
Hall HM, Banerjee S, McGuire DK (2011) Variability of clopidogrel response in patients with type 2 diabetes mellitus. Diab Vasc Dis Res 8(4):245–253
Erlinge D, Varenhorst C, Braun OO, James S, Winters KJ, Jakubowski JA et al (2008) Patients with poor responsiveness to thienopyridine treatment or with diabetes have lower levels of circulating active metabolite, but their platelets respond normally to active metabolite added ex vivo. J Am Coll Cardiol 52(24):1968–1977
Schneider DJ (2009) Factors contributing to increased platelet reactivity in people with diabetes. Diabetes Care 32(4):525–527
Bhatt DL (2008) What makes platelets angry: diabetes, fibrinogen, obesity, and impaired response to antiplatelet therapy? J Am Coll Cardiol 52(13):1060–1061
Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC et al (2015) Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 372(19):1791–1800
Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG et al (2014) Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 371(23):2155–2166
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
F. Krackhardt and M. Noutsias have received lecturing fees from B. Braun Melsungen AG . M. Waliszewski and M. Boxberger are currently full-time employed at Medical Scientific Affairs, B. Braun Melsungen AG. J. Rischner, C. Piot, M. Pansieri, F.L. Ruiz-Poveda, X.F. Ríos, and B. Kherad declare that they have no competing interests.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975 (in its most recently amended version). Informed consent was obtained from all patients included in the study.
Additional information
Contributions. All authors were involved in the data analysis plan and interpretation, drafting the article, critical revision, and the final approval.
Rights and permissions
About this article
Cite this article
Krackhardt, F., Waliszewski, M., Rischner, J. et al. Nine-month clinical outcomes in patients with diabetes treated with polymer-free sirolimus-eluting stents and 6‑month vs. 12‑month dual-antiplatelet therapy (DAPT). Herz 44, 433–439 (2019). https://doi.org/10.1007/s00059-017-4675-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-017-4675-x