Abstract
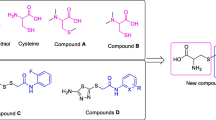
Urease catalyses the hydrolysis of urea to ammonia and carbon dioxide. This enzyme is important in the virulence of several human pathogens and urease activity in soil can cleave urea fertilisers prematurely, leading to waste of agricultural nitrogen. A series of arylsulfonylurea-glucosamine hybrid compounds were synthesised. Reaction of arylsulfonamides with phenyl chloroformate and 4-dimethylaminopyridine gave either phenyl N-(2,4-arylsulfonyl)carbamate 4-dimethylaminopyridinium salts or N-(4-arylsulfonyl)-4-dimethylaminopyridinium-1-carboxamide inner salts, depending on the substitution on the arylsulfonamide. Both types of intermediates, gave ester-protected arylsulfonylurea-glucosamines, when treated with 1,3,4,6-tetra-O-acetylglucosamine. Simple methanolysis gave the arylsulfonylurea-glucosamine hybrids as interconverting mixtures of anomers. Both the O-acetyl intermediates and the target arylsulfonylurea-glucosamines inhibited jack-bean urease with IC50 10–36 μM. This narrow range of values precluded the determination of structure-activity relationships and docking studies suggested several different optimum docking poses for the various analogues. No analogues showed radical-scavenging activity. Several compounds showed modest cytotoxic activity against renal carcinoma cells in the NCI 60-cell-line screen.





Similar content being viewed by others
References
Callahan BP, Yuan Y, Wolfenden R. The burden borne by urease. J Am Chem Soc. 2005;127:10828–9. https://doi.org/10.1021/ja0525399
Rutherford JC. The emerging role of urease as a general microbial virulence factor. PLoS Pathogens. 2014;10:e1004062 https://doi.org/10.1371/journal.ppat.1004062
Song W-Q, Liu M-L, Li S-Y, Xiao Z-P. Recent efforts in the discovery of urease inhibitor identifications. Curr Top Med Chem. 2022;22:95–7. https://doi.org/10.2174/1568026621666211129095441
Svane S, Sigurdarson JJ, Finkenwirth F, Eitinger T, Karring H. Inhibition of urease activity by different compounds provides insight into the modulation and association of bacterial nickel import and ureolysis. Sci Rep. 2020;10:8503 https://doi.org/10.1038/s41598-020-65107-9
Sohrabi M, Nazari Montazer M, Farid SM, Tanideh N, Dianatpour M, Moazzam A, et al. Design and synthesis of novel nitrothiazolacetamide conjugated to different thioquinazolinone derivatives as anti-urease agents. Sci Rep. 2022;12:2003 https://doi.org/10.1038/s41598-022-05736-4
Hamad A, Khan MA, Rahman KM, Ahmad I, Ul-Haq Z, Khan S, et al. Development of sulfonamide-based Schiff bases targeting urease inhibition: Synthesis, characterization, inhibitory activity assessment, molecular docking and ADME studies. Bioorg Chem. 2020;102:104057 https://doi.org/10.1016/j.bioorg.2020.104057
Bury‐Moné S, Skouloubris S, Labigne A, De Reuse H. The Helicobacter pylori UreI protein: role in adaptation to acidity and identification of residues essential for its activity and for acid activation. Mol Microbiol. 2001;42:1021–34. https://doi.org/10.1046/j.1365-2958.2001.02689.x
Paczosa MK, Mecsas J. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev. 2016;80:629–61. https://doi.org/10.1128/mmbr.00078-15
Rosenstein IJ, Hamilton-Miller J, Musher DM. Inhibitors of urease as chemotherapeutic agents. CRC Crit Rev Microbiol. 1984;11:1–12
Kafarski P, Talma M. Recent advances in design of new urease inhibitors: A review. J Adv Res. 2018;13:101–12. https://doi.org/10.1016/j.jare.2018.01.007
Noreen M, Rasool N, Gull Y, Zubair M, Mahmood T, Ayub K, et al. Synthesis, density functional theory (DFT), urease inhibition and antimicrobial activities of 5-aryl thiophenes bearing sulphonylacetamide moieties. Mol. 2015;20:19914–28. https://doi.org/10.3390/molecules201119661
Bailie N, Osborne C, Leininger J, Fletcher T, Johnston S, Ogburn P, et al. Teratogenic effect of acetohydroxamic acid in clinically normal beagles. Am J Vet Res. 1986;47:2604–11
Prakash O, Bachan Upadhyay LS. Acetohydroxamate inhibition of the activity of urease from dehusked seeds of water melon (Citrullus vulgaris). J Enz Inh Med Chem. 2004;19:381–7. https://doi.org/10.1080/14756360409162454
Shi W-K, Deng R-C, Wang P-F, Yue Q-Q, Liu Q, Ding K-L, et al. 3-Arylpropionylhydroxamic acid derivatives as Helicobacter pylori urease inhibitors: Synthesis, molecular docking and biological evaluation. Bioorg Med Chem. 2016;24:4519–27. https://doi.org/10.1016/j.bmc.2016.07.052
Mamidala R, Bhimathati SRS, Vema A. Discovery of Novel Dihydropyrimidine and hydroxamic acid hybrids as potent Helicobacter pylori Urease inhibitors. Bioorg Chem. 2021;114:105010 https://doi.org/10.1016/j.bioorg.2021.105010
Rezaei EB, Abedinifar F, Azizian H, Montazer MN, Asadi M, Hosseini S, et al. Design, synthesis, and evaluation of metronidazole-1, 2, 3-triazole derivatives as potent urease inhibitors. Chem Pap. 2021;75:4217–26
Khan M, Khan KM, Parveen S, Shaikh M, Fatima N, Choudhary MI. Syntheses, in vitro urease inhibitory activities of urea and thiourea derivatives of tryptamine, their molecular docking and cytotoxic studies. Bioorg Chem. 2019;83:595–10. https://doi.org/10.1007/s11696-021-01653-4
Ahmed A, Saeed A, Ali OM, El-Bahy ZM, Channar PA, Khurshid A, et al. Exploring amantadine derivatives as urease inhibitors: Molecular docking and structure–activity relationship (SAR) studies. Mol. 2021;26:7150 https://doi.org/10.3390/molecules26237150
Todd MJ, Hausinger RP. Fluoride inhibition of Klebsiella aerogenes urease: mechanistic implications of a pseudo-uncompetitive, slow-binding inhibitor. Biochem. 2000;39:5389–96. https://doi.org/10.1021/bi992287m
Benini S, Cianci M, Mazzei L, Ciurli S. Fluoride inhibition of Sporosarcina pasteurii urease: structure and thermodynamics. JBIC J Biol Inog Chem. 2014;19:1243–61. https://doi.org/10.1007/s00775-014-1182-x
Mohammed A, Suaifan GARY, Shehadeh MB, Okechukwu PN. Design, synthesis and biological evaluation of 1, 8-naphthyridine glucosamine conjugates as antimicrobial agents. Drug Dev Res. 2019;80:179–86. https://doi.org/10.1002/ddr.21508
Suaifan GA, Shehadeh MB, Darwish RM, Al-Ijel H, Abbate V. Design, synthesis and in vivo evaluation of novel glycosylated sulfonylureas as antihyperglycemic agents. Mol. 2015;20:20063–78. https://doi.org/10.3390/molecules201119676
Mohammed AA, Suaifan GA, Shehadeh MB, Okechukwu PN. Design, synthesis and antimicrobial evaluation of novel glycosylated-fluoroquinolones derivatives. Eur J Med Chem. 2020;202:112513 https://doi.org/10.1016/j.ejmech.2020.112513
Zahedipour F, Dalirfardouei R, Karimi G, Jamialahmadi K. Molecular mechanisms of anticancer effects of Glucosamine. Biomed Pharmacor. 2017;95:1051–8. https://doi.org/10.1016/j.biopha.2017.08.12226
Chen Q, Yang F, Du Y. Synthesis of a C3-symmetric (1→6)-N-acetyl-β-D-glucosamine octadecasaccharide using click chemistry. Car Res. 2005;340:2476–82. https://doi.org/10.1016/j.carres.2005.08.013
Konda S, Raparthi S, Bhaskar K, Munaganti RK, Guguloth V, Nagarapu L, et al. Synthesis and antimicrobial activity of novel benzoxazine sulfonamide derivatives. Bioorg Med Chem Lett. 2015;25:1643–6. https://doi.org/10.1016/j.bmcl.2015.01.026
Lal J, Gupta SK, Thavaselvam D, Agarwal DD. Biological activity, design, synthesis and structure activity relationship of some novel derivatives of curcumin containing sulfonamides. Euro J Med Chem. 2013;64:579–88. https://doi.org/10.1016/j.ejmech.2013.03.012
Abbas A, Murtaza S, Tahir MN, Shamim S, Sirajuddin M, Rana UA, et al. Synthesis, antioxidant, enzyme inhibition and DNA binding studies of novel N-benzylated derivatives of sulfonamide. J Mol Struct. 2016;1117:269–75. https://doi.org/10.1016/j.molstruc.2016.03.066
Kennedy JF, Thorley M. Pharmaceutical Substances. In: Kleeman A, Engel J, Kutscher B, Reichert George D, eds. Bioseparation. 3rd Ed. Stuttgart/New York: Thiele Verlag; 1999. p. 2286. 10.1023/A:1008114712553
Suaifan GARY, Goodyer CL, Threadgill MD. Synthesis of N-(methoxycarbonylthienylmethyl) thioureas and evaluation of their interaction with inducible and neuronal nitric oxide synthase. Molecules. 2010;15:3121–34
Ghorab MM, Alsaid MS, El-Gaby MS, Safwat NA, Elaasser MM, Soliman AM. Biological evaluation of some new N-(2, 6-dimethoxypyrimidinyl) thioureido benzenesulfonamide derivatives as potential antimicrobial and anticancer agents. Euro J Med Chem. 2016;124:299–10. https://doi.org/10.1016/j.ejmech.2016.08.060
Wan Y, Fang G, Chen H, Deng X, Tang Z. Sulfonamide derivatives as potential anti-cancer agents and their SARs elucidation. Euro J Med Chem. 2021;226:113837 https://doi.org/10.1016/j.ejmech.2021.113837
Ma T, Fuld AD, Rigas JR, Hagey AE, Gordon GB, Dmitrovsky E, et al. A phase I trial and in vitro studies combining ABT-751 with carboplatin in previously treated non-small cell lung cancer patients. Chemotherapy. 2012;58:321–9. https://doi.org/10.1159/000343165
Rauf MK, Badshah A, Gielen M, Ebihara M, de Vos D. Ahmed S. Synthesis, structural characterization and in vitro cytotoxicity and anti-bacterial activity of some copper (I) complexes with N, N′-disubstituted thioureas. J Inorg Biochem. 2009;103:1135–44. https://doi.org/10.1016/j.jinorgbio.2009.05.014
Yaseen S, Rauf MK, Zaib S, Badshah A, Tahir MN, Ali MI, et al. Synthesis, characterization and urease inhibition, in vitro anticancer and antileishmanial studies of Co (III) complexes with N, N, N′-trisubstituted acylthioureas. Inorganica Chim. 2016;443:69–77. https://doi.org/10.1016/j.ica.2015.12.027
Myszka H, Bednarczyk D, Najder M, Kaca W. Synthesis and induction of apoptosis in B cell chronic leukemia by diosgenyl 2-amino-2-deoxy-β-D-glucopyranoside hydrochloride and its derivatives. Carbohyd Res. 2003;338:133–41. https://doi.org/10.1016/S0008-6215(02)00407-X
Weatherburn M. Phenol-hypochlorite reaction for determination of ammonia. Anal Chem. 1967;39:971–4. https://doi.org/10.1021/ac60252a045
Benini S, Rypniewski WR, Wilson KS, Miletti S, Ciurli S, Mangani S. The complex of Bacillus pasteurii urease with acetohydroxamate anion from X-ray data at 1.55Å resolution. J biol Inorg Chem. 2000;5:110–8. https://doi.org/10.1007/s007750050014
Pearson MA, Michel LO, Hausinger RP, Karplus PA. Structures of Cys319 variants and acetohydroxamate-inhibited Klebsiella aerogenes urease. Biochem. 1997;36:8164–72. https://doi.org/10.1021/bi970514j
Saeed A, Larik FA, Channar PA, Mehfooz H, Ashraf MH, Abbas Q, et al. An expedient synthesis of N‐(1‐(5‐mercapto‐4‐((substituted benzylidene) amino)‐4H‐1, 2, 4‐triazol‐3‐yl)‐2‐phenylethyl) benzamides as jack bean urease inhibitors and free radical scavengers: Kinetic mechanism and molecular docking studies. Chem Bio Drug Des. 2017;90:764–77. https://doi.org/10.1111/cbdd.12998
Channar PA, Saeed A, Albericio F, Larik FA, Abbas Q, Hassan M, et al. Sulfonamide-linked ciprofloxacin, sulfadiazine and amantadine derivatives as a novel class of inhibitors of jack bean urease; synthesis, kinetic mechanism and molecular docking. Mol. 2017;22:1352 https://doi.org/10.3390/molecules22081352
Rashid U, Rahim F, Taha M, Arshad M, Ullah H, Mahmood T, et al. Synthesis of 2-acylated and sulfonated 4-hydroxycoumarins: in vitro urease inhibition and molecular docking studies. Bioorg Chem. 2016;66:111–6. https://doi.org/10.1016/j.bioorg.2016.04.005
Liu H, Wang Y, Lv M, Luo Y, Liu B-M, Huang Y, et al. Flavonoid analogues as urease inhibitors: Synthesis, biological evaluation, molecular docking studies and in-silico ADME evaluation. Bioorg Chem. 2020;105:104370 https://doi.org/10.1016/j.bioorg.2020.104370
Moghimi S, Goli‐Garmroodi F, Allahyari‐Devin M, Pilali H, Hassanzadeh M, Mahernia S, et al. Synthesis, evaluation, and molecular docking studies of aryl urea‐triazole‐based derivatives as anti‐urease agents. Arch Pharm. 2018;351:1800005 https://doi.org/10.1002/ardp.201800005
Abid O-U-R, Babar TM, Ali FI, Ahmed S, Wadood A, Rama NH, et al. Identification of novel urease inhibitors by high-throughput virtual and in vitro screening. ACS Med Chem Lett. 2010;1:145–9. https://doi.org/10.1021/ml100068u
Sączewski F, Kornicka A, Brzozowski Z. 4-Dimethylaminopyridinium carbamoylides as stable and non-hazardous substitutes of arylsulfonyl and heteroaryl isocyanates. Green Chem. 2006;8:647–56. https://doi.org/10.1039/B604376C
Sączewski F, Kuchnio A, Samsel M, Łobocka M, Kiedrowska A, Lisewska K, et al. Synthesis of novel aryl (heteroaryl) sulfonyl ureas of possible biological interest. Molecules. 2010;15:1113–26. https://doi.org/10.3390/molecules15031113
Khan KM, Iqbal S, Lodhi MA, Maharvi GM, Choudhary MI, Perveen S. Biscoumarin: new class of urease inhibitors; economical synthesis and activity. Bioorg Med Chem. 2004;12:1963–8. https://doi.org/10.1016/j.bmc.2004.01.010
Begum A, Choudhary M, Betzel C. The first Jack bean urease (Canavalia ensiformis) complex obtained at 1.52 resolution. Protein Data Bank. 2012. https://doi.org/10.2210/pdb4H9M/pdb
Biovia DS. Discovery Studio Visualizer; v21. 1.0. San Diego. CA, USA: 20298 Dassault Systèmes; 2021.
Welsch ME, Snyder SA, Stockwell BR. Privileged scaffolds for library design and drug discovery. Curr Opi Chem Biol. 2010;14:347–61. https://doi.org/10.1016/j.cbpa.2010.02.018
Acknowledgements
The authors would like to acknowledge the Deanship of the Scientific Research for financial support [Grant numbers 2213, 2460] and the support of Dr. Panjwani Center for Molecular Medicine and Drug Research-Pakistan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Disclosures related to phenylsulfonylurea derivatives of 2-amino-2-deoxy-D-glucopyranose derivatives, their method of preparation, and the use thereof has been registered for patent (Reg. No. PCT/JO2022/050010).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Suaifan, G.A.R.Y., Shehadeh, M., Tahboub, D. et al. Synthesis, urease inhibitory and anticancer evaluation of glucosamine-sulfonylurea conjugates. Med Chem Res 33, 663–676 (2024). https://doi.org/10.1007/s00044-024-03208-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-024-03208-0