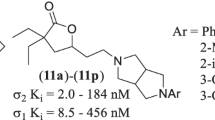
Abstract
The sigma-2 (σ2) receptor has been linked to several diseases and conditions including cancer, neuropathic drug addiction, Alzheimer’s disease, Parkinson’s disease, traumatic brain injury, Niemann-Pick disease, schizophrenia, depression, and anxiety. Targeting σ2 as a means of treating these diseases and conditions has been the subject of intense research, and several clinical trials have been launched to determine the real-world therapeutic utility of this target. Herein, we report the identification of a novel, semirigid series of functionalized 3,3-dialkyl-γ-butyrolactone σ2 ligands, containing piperazine bioisosteres. The compounds were evaluated using a variety of in vitro assays to determine affinity for sigma receptors, σ1/ σ2 selectivity profiles, aqueous solubility, and mouse liver microsome stability.






Similar content being viewed by others
References
Martin WR, Eades CE, Thompson JA, Huppler RE. The effects of morphine and nalorphine-like drugs in the non-dependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–32
Bowen WD, de Costa BR, Hellewell SB, Walker JM, Rice KC. [3H]-(+)-Pentazocine: a potent and highly selective benzomorphan-based probe for sigma-1 receptors. Mol Neuropharmacol. 1993;3:117–26
Hellewell SB, Bowen WD. A sigma-like binding site in rat pheochromocytoma (PC12) cells: decreased affinity for (+)-benzomorphans and lower molecular weight suggest a different sigma receptor form from that of guinea pig brain. Brain Res. 1990;527:244–53. https://doi.org/10.1016/0006-8993(90)91143-5
Alon A, Schmidt HR, Wood MD, Sahn JJ, Martin SF, Krusea AC. Identification of the gene that codes for the σ2 receptor. Proc. Natl. Acad. Sci. USA. 2017;114:7160–5. https://doi.org/10.1073/pnas.1705154114
Alon A, Lyu J, Braz JM, Tummino TA, Craik V, O’Meara MJ, Webb CM, Radchenko DS, Moroz YS, Huang XP, Liu Y, Roth BL, Irwin JJ, Basbaum AI, Shoichet BK, Kruse AC. Structures of the σ2 receptor enable docking for bioactive ligand discovery. Nature. 2021;600:759–64. https://doi.org/10.1038/s41586-021-04175-x
Son KN, Lee H, Shah D, Kalmodia S, Miller RC, Ali M, Balasubramaniam A, Cologna SM, Kong H, Shukla D, Aakalu VK. Histatin-1 is an endogenous ligand of the sigma-2 receptor. FEBS J. 2021;288:6815–27. https://doi.org/10.1111/febs.16108
Ebrahimi-Fakhar D, Wahlster L, Bartz F, Werenbeck-Ueding J, Praggastis M, Zhang J, Joggerst-Thomalla B, Theiss S, Grimm D, Ory DS, Runz H. Reduction of TMEM97 increases NPC1 protein levels and restores cholesterol trafficking in Niemann-pick type C1 disease cells. Hum Mol Genet. 2016;25:3588–99. https://doi.org/10.1093/hmg/ddw204
Sorbi C, Belluti S, Atene CG, Marocchi F, Linciano P, Roy N, Paradiso E, Casarini L, Ronsisvalle S, Zanocco-Marani T, Brasili L, Lanfrancone L, Imbriano C, Di Rocco G, Franchini S. BS148 Reduces the Aggressiveness of Metastatic Melanoma via Sigma-2 Receptor Targeting. Int J Mol Sci. 2023;24:9684. https://doi.org/10.3390/ijms24119684
Abatematteo FS, Niso M, Lacivita E, Abate C. σ2 Receptor and Its Role in Cancer with Focus on a MultiTarget Directed Ligand (MTDL) Approach. Molecules. 2021;26:3743
Shoghi KI, Xu J, Su Y, He J, Rowland D, Yan Y, Garbow JR, Tu Z, Jones LA, Higashikubo R, Wheeler KT, Lubet RA, Mach RH, You M. Quantitative receptor-based imaging of tumor proliferation with the sigma-2 ligand [(18)F]ISO-1. PLoS One. 2013;8:e74188. https://doi.org/10.1371/journal.pone.0074188
McDonald ES, Doot RK, Young AJ, Schubert EK, Tchou J, Pryma DA, Farwell MD, Nayak A, Ziober A, Feldman MD, DeMichele A, Clark AS, Shah PD, Lee H, Carlin SD, Mach RH, Mankoff DA. Breast Cancer 18F-ISO-1 Uptake as a Marker of Proliferation Status. J Nucl Med. 2020;61:665–70. https://doi.org/10.2967/jnumed.119.232363
Li Y, Xie X, Liao S, Zeng Z, Li S, Xie B, Huang Q, Zhou H, Zhou C, Lin J, Huang Y, Xu D. A011, a novel small-molecule ligand of σ2 receptor, potently suppresses breast cancer progression via endoplasmic reticulum stress and autophagy. Biomed Pharmacother. 2022;152:113232. https://doi.org/10.1016/j.biopha.2022.113232
Sahn JJ, Mejia GL, Ray PR, Martin SF, Price TJ. Sigma 2 Receptor/Tmem97 Agonists Produce Long Lasting Antineuropathic Pain Effects in Mice. ACS Chem Neurosci. 2017;8:1801–11. https://doi.org/10.1021/acschemneuro.7b00200
Wilson LL, Alleyne AR, Eans SO, Cirino TJ, Stacy HM, Mottinelli M, Intagliata S, McCurdy CR, McLaughlin JP. Characterization of CM-398, a Novel Selective Sigma-2 Receptor Ligand, as a Potential Therapeutic for Neuropathic Pain. Molecules. 2022;27:3617. https://doi.org/10.3390/molecules27113617
Rishton GM, Look GC, Ni ZJ, Zhang J, Wang Y, Huang Y, Wu X, Izzo NJ, LaBarbera KM, Limegrover CS, Rehak C, Yurko R, Catalano SM. Discovery of Investigational Drug CT1812, an Antagonist of the Sigma-2 Receptor Complex for Alzheimer’s Disease. ACS Med Chem Lett. 2021;12:1389–95. https://doi.org/10.1021/acsmedchemlett.1c00048
Limegrover CS, Yurko R, Izzo NJ, LaBarbera KM, Rehak C, Look G, Rishton G, Safferstein H, Catalano SM. Sigma-2 receptor antagonists rescue neuronal dysfunction induced by Parkinson’s patient brain-derived α-synuclein. J Neurosci Res. 2021;99:1161–76. https://doi.org/10.1002/jnr.24782. https://clinicaltrials.gov/ct2/show/NCT04735536?term=CT1812&draw=2&rank=1
Vázquez-Rosa E, Watson MR, Sahn JJ, Hodges TR, Schroeder RE, Cintrón-Pérez CJ, Shin MK, Yin TC, Emery JL, Martin SF, Liebl DJ, Pieper AA. Neuroprotective Efficacy of a Sigma 2 Receptor/TMEM97 Modulator (DKR-1677) after Traumatic Brain Injury. ACS Chem Neurosci. 2019;10:1595–602. https://doi.org/10.1021/acschemneuro.8b00543
Guo L, Zhen X. Sigma-2 receptor ligands: neurobiological effects. Curr Med Chem. 2015;22:989–1003. https://doi.org/10.2174/0929867322666150114163607
Intagliata S, Alsharif WF, Mesangeau C, Fazio N, Seminerio M, Xu YT, Matsumoto RR, McCurdy CR. Benzimidazolone-based selective σ2 receptor ligands: Synthesis and pharmacological evaluation. Eur J Med Chem. 2019;165:250–7. https://doi.org/10.1016/j.ejmech.2019.01.019
Blass BE, Gao R, Blattner KM, Gordon JC, Pippin DA, Canney DJ. Design, synthesis, and evaluation of novel, selective γ-butyrolactones sigma-2 ligands. Med Chem Res. 2021;30:1713–27. https://doi.org/10.1007/s00044-021-02771-0
Blass BE, Basic Principles of Drug Discovery and Development second edition, Academic Press, 2021, 5, 257–303. https://doi.org/10.1016/B978-0-12-817214-8.00005-1
Blass BE, Blattner KM, Gordon JC, Elokely KM, Pippin DA, Canney DJ. Discovery of selective, functionalized 5-(2-(5-arylhexahydropyrrolo[3,4-c]pyrrol-2(1h)-yl)ethyl)-gama-butyrolactone sigma-2 ligands. Tetrahedron Lett. 2009;50:5914–6
Canney DJ, Blass BE, Gao R, Abou-Ghabia M. Novel 5-hydroxytryptamine receptor 7 activity modulators and their method of use, WO2014164756, 2014
Canney DJ, Blass BE, Blattner KM. 5-Hydroxytryptamine receptor 7 modulators and their use as therapeutic agents, WO2018175190, 2018
Cheng HC. The power issue: determination of KB or Ki from IC50. A closer look at the Cheng-Prusoff equation, the Schild plot and related power equations. J Pharmacol Toxicol Methods. 2001;46:61–71
Yang J, Jamei M, Yeo KR, Rostami-Hodjegan A, Tucker GT. “Misuse of the well-stirred model of hepatic drug clearance.”. Drug Metab Disposition. 2007;35:501–2
McMasters DR, Torres RA, Crathern SJ, Dooney D, Nachbar RB, Sheridan RP, Korzekwa KR. Inhibition of recombinant cytochrome P450 isoforms 2D6 and 2C9 by diverse drug-like molecules. J Med Chem. 2007;50:3205–13. https://doi.org/10.1021/jm0700060
Acknowledgements
Ki determinations for compound binding to σ1, and σ2 were generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program, Contract #HHSN-271-2013-00017-C (NIMH PDSP). The NIMH PDSP is directed by Bryan L. Roth at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscoll at NIMH, Bethesda MD, USA. For experimental details please refer to the PDSP web site https://pdsp.unc.edu/ims/investigator/web/. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. TPSA and cLogP values were generated using the Dotmatics software suite (Dotmatics LLC The Old Monastery, Windhill Bishops, Stortford Herts, CW23 2ND UK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Drs. Blass and Canney both have equity interests in Praeventix LLC, which have been reviewed and approved by Temple University in accordance with its conflict of interest policies. Questions regarding this interest may be directed to the Temple University Conflict of Interest Program. No other author has reported conflicts of interest to disclose at the time of publication.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Blass, B.E., Gao, R., Blattner, K.M. et al. Increased rigidity and bioisosteric replacement in the design, synthesis and preliminary evaluation of novel, functionalized 3,3-dialkyl-γ-butyrolactones as sigma-2 ligands. Med Chem Res 33, 287–297 (2024). https://doi.org/10.1007/s00044-023-03182-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-023-03182-z