Abstract
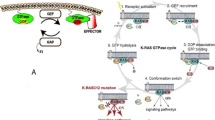
Farnesyltransferase (FTase) is a key enzyme that catalyzes the farnesylation of Ras protein. It is used to bind RAS protein to plasma membrane to complete signal transduction. Ras has been shown to be closely related to the development of many cancers. In recent years, FTase has been studied more deeply as an anticancer target. And more and more novel FTase inhibitors have been reported for the treatment of pancreatic cancer, lung cancer, colon cancer, HGPS, PL and so on. This review summarizes the structural features and biological activities of various novel FTase inhibitors reported since 2013. The reported novel FTase inhibitors were reclassified from the perspective of structure, their current tumor antagonism potential was summarized, and their current challenges and future development trends were further analyzed.









Similar content being viewed by others
References
Gibbs JB, Oliff A. Pharmaceutical research in molecular oncology. Cell. 1994;79:193–8.
Wang JJ, Lei KF, Han F. Tumor microenvironment: recent advances in various cancer treatments. Eur Rev Med Pharm Sci. 2018;22:3855–64.
Moodie SA, Wolfman A. The 3Rs of life: Ras, Raf and growth regulation. Trends Genet. 1994;10:44–48.
Chen S, Li F, Xu D, et al. The Function of RAS Mutation in Cancer and Advances in its Drug Research. Curr Pharm Des. 2019;25:1105–14.
Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22.
Dai W, Xie S, Chen C, et al. Ras sumoylation in cell signaling and transformation. Semin Cancer Biol. 2021;76:301–9.
Dunnett-Kane V, Nicola P, Blackhall F, et al. Mechanisms of Resistance to KRASG12C Inhibitors. Cancers. 2021;13:151.
Hobbs GA, Der CJ, Rossman KL. RAS isoforms and mutations in cancer at a glance. J Cell Sci. 2016;129:1287–92.
Rowinsky EK, Windle JJ, Von Hoff DD. Ras protein farnesyltransferase: A strategic target for anticancer therapeutic development. J Clin Oncol. 1999;17:3631–52.
Sebti SM. Protein farnesylation: implications for normal physiology, malignant transformation, and cancer therapy. Cancer Cell. 2005;7:297–300.
Basso AD, Kirschmeier P, Bishop WR. Lipid posttranslational modifications. Farnesyl transferase inhibitors. J Lipid Res. 2006;47:15–31.
Lobell RB. Prenylation of Ras GTPase superfamily proteins and their function in immunobiology. Adv Immunol. 1998;68:145–89.
Silva LR, da Silva-Júnior EF. Inhibiting the “Undruggable” RAS/Farnesyltransferase (FTase) Cancer Target by Manumycin-related Natural Products. Curr Med Chem. 2022;29:189–11.
Wang Q, Chen F, Liu P, et al. Scaffold-based analysis of nonpeptide oncogenic FTase inhibitors using multiple similarity matching, binding affinity scoring and enzyme inhibition assay. J Mol Graph Model. 2021;105:107898.
Jones HA, Hahn SM, Bernhard E, et al. Ras inhibitors and radiation therapy. Semin Radiat Oncol. 2001;11:328–37.
Nam NH, Parang K. Current targets for anticancer drug discovery. Curr Drug Targets. 2003;4:159–79.
Dhillon S. Lonafarnib: First Approval [published correction appears in Drugs. 2021 Apr;81(5):619]. Drugs. 2021;81:283–9.
Kohl NE. Farnesyltransferase inhibitors. Preclinical development. Ann NY Acad Sci. 1999;886:91–102.
Wang J, Yao X, Huang J. New tricks for human farnesyltransferase inhibitor: cancer and beyond. Medchemcomm. 2017;8:841–54.
Cho KN, Lee KI. Chemistry and biology of Ras farnesyltransferase. Arch Pharm Res. 2002;25:759–69.
Morgillo F, Lee HY. Lonafarnib in cancer therapy. Expert Opin Investig Drugs. 2006;15:709–19.
Mullard A. The FDA approves a first farnesyltransferase inhibitor. Nat Rev Drug Discov. 2021;20:8.
George Njoroge F, Taveras AG, Kelly J, et al. -4-[2-[4-(8-Chloro-3,10-dibromo-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]- pyridin-11(R)-yl)-1-piperidinyl]-2-oxo-ethyl]-1-piperidinecarboxamide (SCH-66336): A Very Potent Farnesyl Protein Transferase Inhibitor as a Novel Antitumor Agent. J Med Chem. 1998;41:4890–4902.
Suzuki M, Jeng LJB, Chefo S, et al. FDA approval summary for lonafarnib (Zokinvy) for the treatment of Hutchinson-Gilford progeria syndrome and processing-deficient progeroid laminopathies. Genet Med. 2023;25:100335.
Arnold R, Vehns E, Randl H, et al. Baricitinib, a JAK-STAT Inhibitor, Reduces the Cellular Toxicity of the Farnesyltransferase Inhibitor Lonafarnib in Progeria Cells. Int J Mol Sci. 2021;22:7474.
Abuhaie CM, Ghinet A, Farce A, et al. Synthesis and biological evaluation of a new series of phenothiazine-containing protein farnesyltransferase inhibitors. Eur J Med Chem. 2013;59:101–10.
Dumitriu GM, Ghinet A, Bîcu E, et al. Peptide chemistry applied to a new family of phenothiazine-containing inhibitors of human farnesyltransferase. Bioorg Med Chem Lett. 2014;24:3180–5.
Dumitriu GM, Bîcu E, Belei D, et al. Phenothiazine-based CaaX competitive inhibitors of human farnesyltransferase bearing a cysteine, methionine, serine or valine moiety as a new family of antitumoral compounds. Bioorg Med Chem Lett. 2015;25:4447–52.
Moise IM, Ghinet A, Belei D, et al. New indolizine-chalcones as potent inhibitors of human farnesyltransferase: Design, synthesis and biological evaluation. Bioorg Med Chem Lett. 2016;26:3730–4.
Moise IM, Bîcu E, Farce A, et al. Indolizine-phenothiazine hybrids as the first dual inhibitors of tubulin polymerization and farnesyltransferase with synergistic antitumor activity. Bioorg Chem. 2020;103:104184.
Dumitriu G-M, Ghinet A, Belei D, et al. Investigation of New Phenothiazine and Carbazole Derivatives as Potential Inhibitors of Human Farnesyltransferase. Lett Drug Des Discov. 2015;12:85–92.
Ghasemi S, Sharifi S, Davaran S, et al. Synthesis and cytotoxicity evaluation of some novel 1-(3-Chlorophenyl) piperazin-2-one derivatives bearing imidazole bioisosteres. Aust J Chem. 2013;66:655–60.
Ghasemi S, Sharifi S, Shahbazi Mojarrad J. Design, Synthesis and Biological Evaluation of Novel Piperazinone Derivatives as Cytotoxic Agents. Adv Pharm Bull. 2020;10:423–9.
Abuhaie CM, Ghinet A, Farce A, et al. Synthesis and biological evaluation of a new series of N-ylides as protein farnesyltransferase inhibitors. Bioorg Med Chem Lett. 2013;23:5887–92.
Dumea C, Belei D, Ghinet A, et al. Novel indolizine derivatives with unprecedented inhibitory activity on human farnesyltransferase. Bioorg Med Chem Lett. 2014;24:5777–81.
Yang L, Liu W, Mei H, et al. Synthesis and biological evaluation of pentanedioic acid derivatives as farnesyltransferase inhibitors. Medchemcomm. 2015;6:671–6.
Lucescu L, Bîcu E, Belei D, et al. Synthesis and biological evaluation of a new class of triazin-triazoles as potential inhibitors of human farnesyltransferase. Res Chem Intermed. 2016;42:1999–2021.
Homerin G, Lipka E, Rigo B, et al. On the discovery of new potent human farnesyltransferase inhibitors: emerging pyroglutamic derivatives. Org Biomol Chem. 2017;15:8110–8.
Jin Y, Li L, Yang Z, et al. The discovery of a novel compound with potent antitumor activity: virtual screening, synthesis, biological evaluation and preliminary mechanism study. Oncotarget. 2017;8:24635–43.
Kazi A, Xiang S, Yang H, et al. Dual Farnesyl and Geranylgeranyl Transferase Inhibitor Thwarts Mutant KRAS-Driven Patient-Derived Pancreatic Tumors. Clin Cancer Res. 2019;25:5984–96.
Tanaka A, Radwan MO, Hamasaki A, et al. A novel inhibitor of farnesyltransferase with a zinc site recognition moiety and a farnesyl group. Bioorg Med Chem Lett. 2017;27:3862–6.
Tsubamoto M, Le TK, Li M, et al. A Guanidyl-Based Bivalent Peptidomimetic Inhibits K-Ras Prenylation and Association with c-Raf. Chemistry. 2019;25:13531–6.
Pesquet A, Marzag H, Knorr M, et al. Access to 3-spiroindolizines containing an isoindole ring through intra-molecular arylation of spiro-N-acyliminium species: a new family of potent farnesyltransferase inhibitors. Org Biomol Chem. 2019;17:2798–808.
Rampogu S, Baek A, Son M, et al. Discovery of Lonafarnib-Like Compounds: Pharmacophore Modeling and Molecular Dynamics Studies. ACS Omega. 2020;5:1773–81.
Safavi A, Ghodousi ES, Ghavamizadeh M, et al. Computational investigation of novel farnesyltransferase inhibitors using 3D-QSAR pharmacophore modeling, virtual screening, molecular docking and molecular dynamics simulation studies: A new insight into cancer treatment. J Mol Struct. 2021;1241: 130667.
Yang W, Wang K, Wu H, et al. Peptide scaffold‐derived peptidomimetic farnesyltransferase inhibitors. J Chin Chem Soc. 2021;68:1778–88.
Pierrick D, Marie H, Adam D, et al. Green synthesis of a new series of pyroglutamides targeting human farnesyltransferase. Sustainable Chem Pharmacy. 2022;30:100894.
Bellesia F, Choi SR, Felluga F, et al. Novel route to chaetomellic acid A and analogues: serendipitous discovery of a more competent FTase inhibitor. Bioorg Med Chem. 2013;21:348–58.
Cadelis MM, Bourguet-Kondracki ML, Dubois J, et al. Discovery and preliminary structure-activity relationship studies on tecomaquinone I and tectol as novel farnesyltransferase and plasmodial inhibitors. Bioorg Med Chem. 2016;24:3102–7.
Kim J, Park M, Choi J, et al. Design, synthesis, and biological evaluation of novel pyrrolo[1,2-a]pyrazine derivatives. Bioorg Med Chem Lett. 2019;29:1350–6.
Lucescu L, Ghinet A, Shova S, et al. Exploring isoxazoles and pyrrolidinones decorated with the 4,6-dimethoxy-1,3,5-triazine unit as human farnesyltransferase inhibitors. Arch Pharm (Weinh). 2019;352:e1800227.
Bukhtiyarova M, Cook EM, Hancock PJ, et al. Discovery of an Anion-Dependent Farnesyltransferase Inhibitor from a Phenotypic Screen. ACS Med Chem Lett. 2020;12:99–106.
Homerin G, Nica AS, Farce A, et al. Ultrasounds-mediated 10-seconds synthesis of chalcones as potential farnesyltransferase inhibitors. Bioorg Med Chem Lett. 2020;30:127149.
Nguyen UT, Goody RS, Alexandrov K. Understanding and exploiting protein prenyltransferases. Chembiochem. 2010;11:1194–201.
Moorthy NS, Sousa SF, Ramos MJ, et al. Farnesyltransferase inhibitors: a comprehensive review based on quantitative structural analysis. Curr Med Chem. 2013;20:4888–923.
Haluska P, Dy GK, Adjei AA. Farnesyl transferase inhibitors as anticancer agents. Eur J Cancer. 2002;38:1685–700.
Agrawal AG, Somani RR. Farnesyltransferase inhibitor as anticancer agent. Mini Rev Med Chem. 2009;9:638–52.
Reiss Y, Goldstein JL, Seabra MC, et al. Inhibition of purified p21ras farnesyl:protein transferase by Cys-AAX tetrapeptides. Cell. 1990;62:81–88.
Tsimberidou AM, Chandhasin C, Kurzrock R. Farnesyltransferase inhibitors: where are we now? Expert Opin Investig Drugs. 2010;19:1569–80.
Fernández-Medarde A, Santos E. Ras in cancer and developmental diseases. Genes Cancer. 2011;2:344–58.
Oikonomou E, Koustas E, Goulielmaki M, et al. BRAF vs RAS oncogenes: are mutations of the same pathway equal? Differential signalling and therapeutic implications. Oncotarget. 2014;5:11752–77.
Park HW, Boduluri SR, Moomaw JF, et al. Crystal structure of protein farnesyltransferase at 2.25 angstrom resolution. Science. 1997;275:1800–4.
Subramanian T, Liu S, Troutman JM, et al. Protein farnesyltransferase-catalyzed isoprenoid transfer to peptide depends on lipid size and shape, not hydrophobicity. Chembiochem. 2008;9:2872–82.
Sousa SF, Fernandes PA, Ramos MJ. Farnesyltransferase inhibitors: a detailed chemical view on an elusive biological problem. Curr Med Chem. 2008;15:1478–92.
Klochkov SG, Neganova ME, Yarla NS, et al. Implications of farnesyltransferase and its inhibitors as a promising strategy for cancer therapy. Semin Cancer Biol. 2019;56:128–34.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No.82204210), the Hebei Natural Science Foundation (NO. H2022406062), the Funded by Science and Technology Project of Hebei Education Department (NO. QN2022161), the 2022 Research Start‐up Fund for High‐level Talents of Chengde Medical University (NO. 202207), and the Chengde Medical University basic research funds special project (NO. KY202315).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xia, Mt., Yu, L., Yan, Zw. et al. FTase inhibitors and cancer: prospects for use in targeted therapies. Med Chem Res 33, 21–35 (2024). https://doi.org/10.1007/s00044-023-03171-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-023-03171-2