Abstract
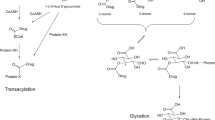
Several carboxylic acid-containing drugs have been withdrawn from clinical use due to adverse drug reactions including idiosyncratic hepatotoxicity and anaphylaxis. Carboxylic acid drugs are increasingly being shown to be metabolized to chemically-reactive acyl-CoA thioester-linked intermediary metabolites that are proposed to potentially mediate these toxicities. Acyl-CoA thioesters possess an electrophilic carbonyl-carbon and are able to undergo non-enzyme catalyzed transacylation reactions with biological nucleophiles leading to covalent binding to protein and drug-protein adduct formation. Such drug-protein adducts are proposed to mediate idiosyncratic drug toxicity reactions to carboxylic acid drugs, similar to the more studied mechanism of chemically-reactive acyl glucuronides potentially mediating carboxylic acid drug toxicity through the formation of immunogenic covalent protein adducts. Carboxylic acid drug acyl-CoA formation is catalyzed by acyl-CoA synthetase enzymes located with highest activity in liver tissue and in mitochondrial, peroxisomal, microsomal, and cytosolic cellular fractions. As is also true for unstable and reactive acyl glucuronide metabolites, the role of reactive acyl-CoA thioester metabolites in mediating idiosyncratic drug toxicity is difficult to assess. Importantly, in comparison to acyl glucuronide formation, results from mechanistic studies have revealed that acyl-CoA thioester formation can be the predominant pathway leading to covalent protein adduct formation in vitro and in vivo. This review includes an examination of the metabolism of carboxylic acid-containing drugs to reactive acyl-CoA thioesters leading to covalent binding to protein and potential implications with regard to carboxylic acid drug toxicity.









Similar content being viewed by others
References
Ballatore C, Huryn DM, Smith AB. Carboxylic acid (bio)isosteres in drug design. ChemMedChem. 2013;8:385–95. https://doi.org/10.1002/cmdc.201200585
Fung M, Thornton A, Mybeck K, Wu JH, Hornbuckle K, Muniz E. Evaluation of the characteristics of safety withdrawal of prescription drugs from world-wide pharmaceutical markets-1960 to 1999. Drug Inf J. 2001;35:293–317. https://doi.org/10.1177/009286150103500134
Zimmerman HJ. Hepatic injury associated with nonsteroidal anti-inflammatory drugs. In: Lewis AJ, Gay GR, eds. Nonsteroidal anti-inflammatory drugs: mechanisms and clinical uses. 2nd ed. New York: Marcel Dekker; 1994. p. 171–94.
Boelsterli UA, Zimmerman HJ, Kretz-Rommel A. Idiosyncratic liver toxicity of nonsteroidal anti-inflammatory drugs: molecular mechanisms and pathology. Crit Rev Toxicol. 1995;25:207–35. https://doi.org/10.3109/10408449509089888
Sánchez-Borges M, Caballero-Fonseca F, Cariles-Hulett A, González-Aveledo L. Hypersensitivity reactions to nonsteroidal anti-inflammatory drugs: an update. Pharmaceuticals. 2010;3:10–18. https://doi.org/10.3390/ph3010010
Reddy JK, Rao MS. Malignant tumors in rats fed nafenopin, a hepatic peroxisome proliferator. J Natl Cancer Inst. 1977;59:1645–50. https://doi.org/10.1093/jnci/59.6.1645
Faed EM. Properties of acyl glucuronides: implications for studies of the pharmacokinetics and metabolism of acidic drugs. Drug Metab Rev. 1984;15:1213–49. https://doi.org/10.3109/03602538409033562
Domínguez-Ortega J, Martínez-Alonso JC, Domínguez-Ortega C, Fuentes MJ, Frades A, Fernández-Colino T. Anaphylaxis to oral furosemide. Allergol et Immunopathol. 2003;31:345–7. https://doi.org/10.1016/S0301-0546(03)79210-6
Bota RG, Ligasan AP, Najdowski TG, Novac A. Acute hypersensitivity syndrome caused by valproic acid: a review of the literature based on a case report. Perm J 2011;15:80–4. https://doi.org/10.7812/TPP/10-14
Kowalski ML, Makowska JS, Blanca M, Bavbek S, Bochenek G, Bousquet P, et al. Hypersensitivity to nonsteroidal anti-inflammatory drugs (NSAIDs) – classification, diagnosis and management: review of the EAACI/ENDA and GA2LEN/HANA. Allergy. 2011;66:818–29. https://doi.org/10.1111/j.1398-9995.2011.02557.x
Boelsterli UA. Xenobiotic acyl glucuronides and acyl-CoA thioesters as protein reactive metabolites with the potential to cause idiosyncratic drug reactions. Curr Drug Metab. 2002;4:3439–50.
Stogniew M, Fenselau C. Electrophilic reactions of acyl-linked glucuronides. Drug Metab Dispos. 1982;10:609–13.
Sallustio BC, Nunthasomboon S, Drogemuller CJ, Knights KM. In vitro covalent binding of nafenopin-CoA to human liver proteins. Toxicol Appl Pharm. 2000;163:176–82. https://doi.org/10.1006/taap.1999.886
Hertz R, Bar-Tana J. The acylation of proteins by xenobiotic amphipathic carboxylic acids in cultured rat hepatocytes. Biochem J. 1988;254:39–44. https://doi.org/10.1042/bj2540039
Langguth HS, Benet LZ. Acyl glucuronides revisited: is the glucuronidation proces a toxification as well as a detoxification mechanism? Drug Metab Rev. 1992;24:5–47. https://doi.org/10.3109/03602539208996289
Porubek DJ, Grillo MP, Baillie TA. The covalent binding of valproic acid and its hepatotoxic metabolite, 2-n-propyl-4-pentenoic acid, in rats and in isolated rat hepatocytes. Drug Metab Dispos. 1989;17:123–30.
Spracklin DK, Thummel KE, Kharasch ED. Human reductive halothane metabolism in vitro is catalyzed by cytochrome P450 2A6 and 3A4. Drug Metab Dispos. 1996;24:976–83.
Levine B. Immunologic mechanisms of penicillin allergy. A haptenic model system for the study of allergic disease in man. N. Engl J Med. 1966;275:1115–25.
Huxtable R. Thiols, disulfides and thioesters. In: Frieden E, editor. Biochemistry of Sulfur. New York: Plenum Press; 1986. p. 230–45.
Dutton GJ. Glucuronidation of drugs and other compounds. Boca Raton, FL: CRC Press; 1980.
Smith PC, Benet LZ, McDonagh AF. Covalent binding of zomepirac glucuronide to proteins: evidence for a Schiff base mechanism. Drug Metab Dispos. 1990;18:639–44.
Ding A, Ojingwa JC, McDonagh AF, Benet LZ. Evidence for covalent binding of acyl glucuronides to serum albumin via an imine mechanism as revealed by tandem mass spectrometry. Proc Natl Acad Sci. 1993;90:3797–801. https://doi.org/10.1073/pnas.90.9.37
Benet LZ, Spahn-Langguth H, Iwakawa S, Volland C, Mizuma T, Mayer S, et al. Predictability of covalent binding of acidic drugs in man. Life Sci. 1993;53:141–46. https://doi.org/10.1016/0024-3205(93)90279-C
Sawamura R, Okudaira, Watanabe K, Murai T, Kobayashi Y, Tachibana M, et al. Predictability of idiosyncratic drug toxicity risk for carboxylic acid-containing drugs based on the chemical instability of acyl glucuronide. Drug Metab Dispos. 2010;38:1857–64. https://doi.org/10.1124/dmd.110.034173
Iwamura A, Ito M, Mitsui H, Hasegawa J, Kosaka K, Kino I, et al. Toxicological evaluation of acyl glucuronides utilizing half-lives, peptide adducts, and immunostimulation assays. Toxicol Vitr. 2015;30:241–9. https://doi.org/10.1016/j.tiv.2015.10.013
Baba A, Yoshioka T. Structure-activity relationships for degradation reaction of 1-β-O-acyl glucuronides: kinetic description and prediction of intrinsic electrophilic reactivity under physiological conditions. Chem Res Toxicol. 2009;22:158–72. https://doi.org/10.1021/tx800292m
Camilleri P, Buch A, Soldo B, Hutt AJ. The influence of physicochemical properties on the reactivity and stability of acyl glucuronides. Xenobiotica. 2018;48:958–72. https://doi.org/10.1080/00498254.2017.1384967
Bradshaw PR, Athersuch TJ, Stachulski AV, Wilson ID. Acyl glucuronide reactivity in perspective. Drug Disco Today. 2020;25:1639–50. https://doi.org/10.1016/j.drudis.2020.07.009
Knights KM. Role of hepatic fatty acid: coenzyme A ligases in the metabolism of xenobiotic carboxylic acids. Clin Exp Pharm Physiol. 1998;25:776–82. https://doi.org/10.1111/j.1440-1681.1998.tb02152.x
Knights KM, Sykes MJ, Miners JO. Amino acid conjugation: contribution to the metabolism and toxicity of xenobiotic carboxylic acids. Expert Opin Drug Metab Toxicol. 2007;3:159–68. https://doi.org/10.1517/17425255.3.2.159
Grillo MP. Drug-S-acyl-glutathione thioesters: synthesis, bioanalytical properties, chemical reactivity, biological formation and degradation. Curr Drug Metab. 2011;12:229–44.
Stern RH. The role of nicotinic acid metabolites in flushing and hepatotoxicity. J Clin Lipido. 2007;3:191–3. https://doi.org/10.1016/j.jacl.2007.04.003
Shirley MA, Guan X, Kaiser DG, Halstead GW, Baillie TA. Taurine conjugation of ibuprofen in humans and in rat liver in vitro. Relationship to metabolic chiral inversion. J Pharm Exp Ther. 1994;269:1166–75.
Tishler SL, Goldman P. Properties and reactions of salicyl-coenzyme A. Biochem Pharm. 1970;19:143–50. https://doi.org/10.1016/0006-2952(70)90335-7
Grillo MP, Benet LZ. Studies on the reactivity of clofibryl-S-acyl-CoA thioester with glutathione in vitro. Drug Metab Dispos. 2002;30:55–62. https://doi.org/10.1124/dmd.30.1.55
Li C, Benet LZ, Grillo MP. Studies on the chemical reactivity of 2-phenylpropionic acid 1-O-acyl glucuronide and S-acyl-CoA thioester metabolites. Chem Res Toxicol. 2002;15:1309–17. https://doi.org/10.1021/tx020013l
Li C, Grillo MP, Benet LZ. In vitro studies on the chemical reactivity of 2,4-dichlorophenoxyacetyl-S-acyl-CoA thioester. Toxicol Appl Pharm. 2003;187:101–9. https://doi.org/10.1016/S0041-008X(02)00043-1
Grillo MP, Lohr MT. Covalent binding of phenylacetic acid to protein in incubations with freshly isolated rat hepatocytes. Drug Metab Dispos. 2009;37:1073–89. https://doi.org/10.1124/dmd.108.026153
Tillander V, Alexson SHE, Cohen DE. Deactivating fatty acids: acyl-CoA thioesterase-mediated control of lipid metabolism. Trends Endrocrinol Metab. 2017;28:473–84. https://doi.org/10.1026/j.tem.2017.03.001
Dickinson RG, Baker PV, King AR. Studies on the reactivity of acyl glucuronides–VII. Salicyl acyl glucuronide reactivity in vitro and covalent binding of salicylic acid to plasma protein of humans taking aspirin. Biochem Pharm. 1994;47:469–76. https://doi.org/10.1016/0006-2952(94)90177-5
Duncan JA, Gilman AG. Autoacylation of G protein alpha subunits. J Biol Chem. 1996;271:23594–600. https://doi.org/10.1074/jbc.271.38.23594
Yamashita A, Watanabe M, Tonegawa T, Sugiura K, Waku K. Acyl-CoA binding and acylation of UDP-glucuronosyltransferase isoforms of rat liver: their effect on enzyme activity. Biochem J. 1995;312:301–8. https://doi.org/10.1042/bj3120301
Goddard AD, Watts A. Regulation of G protein-coupled receptors by palmitoylation and cholesterol. BMC Biol. 2012;10:27.
Trefely S, Lovell CD, Snyder NW, Wellen KE. Compartmentalised acyl-CoA metabolism and roles in chromatin regulation. Mol Metab. 2020;38:100941. https://doi.org/10.1016/j.molmet.2020.01.005
Wagner GR, Payne RM. Widespread and enzyme-independent Nε-acetylation and Nε-succinylation of proteins in the chemical conditions of the mitochondrial matrix. J Biol Chem. 2013;288:29036–45. https://doi.org/10.1074/jbc.M113.486753
Wagner GR, Bhatt DP, O’Connell M, Thompson W, Dubois LG, Backos DS, et al. A class of reactive acyl-CoA species reveals the non-enzymatic origins of protein acylation. Cell Metab. 2017;25:823–37. https://doi.org/10.1016/j.cmet.2017.03.006
Kulkarni RA, Worth AJ, Zengeya TT, Shrimp JH, Garlick JM, Robert AM, et al. Discovering targets of non-enzymatic acylation by thioester reactivity profiling. Cell Chem Biol. 2017;24:231–42. https://doi.org/10.1016/j.chembiol.2017.01.002
James AM, Hoogewijs K, Logan A, Hall AR, Ding S, Fearnley IM, et al. Non-enzymatic N-acetylation of lysine residues by acetyl-CoA often occurs via a proximal S-acetylated thiol intermediate sensitive to glyoxalase II. Cell Rep. 2017;18:2105–12. https://doi.org/10.1016/j.celrep.2017.02.018
Weinert BT, Moustafa T, Iesmantavicius V, Zechner R, Choudhary C. Analysis of acetylation stoichiometry suggests that Sirt3 repairs nonenzymatic acetylation lesions. EMBO J. 2015;34:2620–32. https://doi.org/10.15252/embj.201591271
Baillie TA, Davis MR. Mass spectrometry in the analysis of glutathione conjugates. Biol Mass Spectrom. 1993;22:319–25. https://doi.org/10.1002/bms.1200220602
Shore LJ, Fenselau C, King AR, Dickinson RG. Characterization and formation of the glutathione conjugate of clofibric acid. Drug Metab Dispos. 1995;23:119–23.
Sidenius U, Skonberg C, Olsen J, Hansen SH. In vitro reactivities of carboxylic acid-CoA thioesters with glutathione. Chem Res Toxicol. 2004;17:75–8. https://doi.org/10.1021/tx034127o
Li C, Olurinde MO, Hodges LM, Hodges LM, Grillo MP, Benet LZ. Covalent binding of 2-phenylpropionyl-S-acyl-CoA thioester to tissue proteins in vitro. Drug Metab Dispos. 2003;31:727–30. https://doi.org/10.1124/dmd.31.6.727
Grillo MP, Hua F. Enantioselective formation of ibuprofen-S-acyl-glutathione in vitro in incubations of ibuprofen with rat hepatocytes. Chem Res Toxicol. 2008;21:1749–59. https://doi.org/10.1021/tx800098h
Darnell M, Breitholtz K, Isin EM, Jurva U, Weidolf L. Significantly different covalent binding of oxidative metabolites, acyl glucuronides, and S-acyl CoA conjugates formed from xenobiotic carboxylic acids in human liver microsomes. Chem Res Toxicol. 2015;28:886–96. https://doi.org/10.1021/tx500514z
Shang J, Tschirret-Guth R, Cancilla M, Samuel K, Chen Q, Chobanian HR, et al. Bioactivation of GPR40 agonist MK-8666: formation of protein adducts in vitro from reactive acyl glucuronide and acyl CoA thioester. Chem Res Toxicol. 2020;33:191–201. https://doi.org/10.1021/acs.chemrestox.9b00226
Knihinicki RD, Williams KM, Day RO. Chiral inversion of 2-arylpropionic acid non-steroidal anti-inflammatory drugs—1: In vitro studies of ibuprofen and flurbiprofen. Biochem Pharm. 1989;15:4389–95. https://doi.org/10.1016/0006-2952(89)90647-3
Knadler MP, Hall SD. Stereoselective arylpropionyl-CoA thioester formation in vitro. Chirality. 1990;2:67–73. https://doi.org/10.1002/chir.530020202
Li C, Benet LZ, Grillo MP. Enantioselective covalent binding of 2-phenylpropionic acid to protein in vitro in rat hepatocytes. Chem Res Toxicol. 2003;15:1480–7. https://doi.org/10.1021/tx025600l
Li C, Grillo MP, Benet LZ. In vivo mechanistic studies on the metabolic activation of 2-phenylpropionic acid in rat. J Pharm Exp Ther. 2003;305:250–6. https://doi.org/10.1124/jpet.102.043174
Knights KM, Addinall TF, Roberts BJ. Enhanced chiral inversion of R-ibuprofen in liver from rats treated with clofibric acid. Biochem Pharm. 1991;41:1775–7. https://doi.org/10.1016/0006-2952(91)90184-7
Li C, Grillo MP, Badagnani I, Fife KL, Benet LZ. Differential effects of fibrates on the metabolic activation of 2-phenylpropionic acid in rats. Drug Metab Dispos. 2008;36:682–7. https://doi.org/10.1124/dmd.107.017764
Baillie TA. Chemically reactive metabolites in drug discovery and development. In: Lee JS, Obach RS, Fisher MB, editors. Drug metabolizing enzymes: cytochrome p450 and other enzymes in drug discovery and development. Lausanne: Fontis Media; 2003. p. 147–54.
Baillie TA. Metabolism and toxicity of drugs. Two decades of progress in industrial drug metabolism. Chem Res Toxicol. 2008;21:129–37. https://doi.org/10.1021/tx7002273
Baillie TA. Metabolic Activation and Associated Drug Toxicity. In: Lee PW, Aizawa H, Gan LL, Prakash C, Zhong D, editors. Handbook of metabolic pathways of xenobiotics, 1st ed. Chichester, UK: John Wiley & Sons, Ltd.; 2014. p. 303–20.
Trub AG, Hirschey MD. Reactive acyl-CoA species (RACS) modify proteins and induce carbon stress. Trends Biochem Sci. 2018;43:369–76. https://doi.org/10.1016/j.tibs.2018.02.002
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Grillo, M.P., Li, C. & Benet, L.Z. Acyl-CoA thioesters as chemically-reactive intermediates of carboxylic acid-containing drugs. Med Chem Res 32, 2058–2070 (2023). https://doi.org/10.1007/s00044-023-03144-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-023-03144-5